Description
The instruction for medical use of medicine
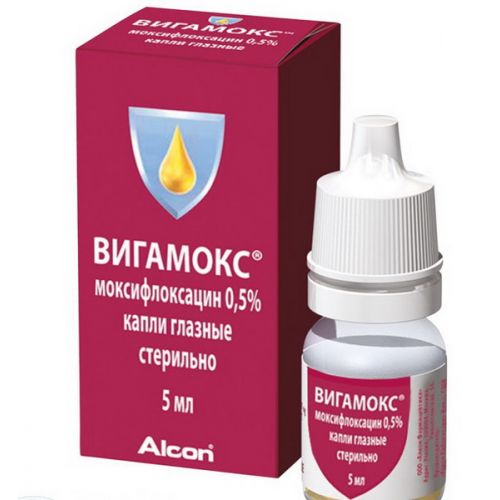
VIGAMOKS
A trade name
of Vigamoks
the International unlicensed
name Moxifloxacin Dosage Form
of the Drop eye 0.5% 5 ml
Structure
of 1 ml contains
active agent of a moksifloksatsin of a hydrochloride of 5.45 mg (5 mg of a moksifloksatsin are equivalent),
excipients: sodium chloride, boric acid, acid chlorohydrogen and/or sodium hydroxide (for adjustment rn), water for injections.
Description
Transparent solution of chartreuse color.
Pharmacotherapeutic group
Drugs for treatment of diseases of eyes. Antibacterial drugs.
Code of automatic telephone exchange S01AX
Pharmacological
Pharmacokinetics At properties topical administration of eye drops of Vigamoks perhaps system absorption of a moksifloksatsin. Elimination half-life of a moksifloksatsin makes 13 hours of plasma.
The pharmacodynamics
Moxifloxacin is the representative of the 4th generation of ftorkhinolonovy connections, and affects a wide range of gram-positive and gram-negative bacteria, atypical microorganisms and anaerobe bacterias.
Inhibits topoisomerase 2 (DNK-girazu) and topoisomerase, responsible for replication, a transcription, restoration and a recombination of DNA of bacteria. The C8-methoxy group of a moksifloksatsin reduces selection of resistant strains of the gram-positive bacteria unlike the C8-H group found in ftorkhinolon of more senior generation. Big replaceable S-7 group of a moksifloksatsin breaks work of hinolonovy receptors of bacteria. Bactericidal concentration of a moksifloksatsin is equal to a thicket or is a little higher than the inhibiting concentration.
Ftorkhinolona, including moxifloxacin, differ on the action from a beta laktamnykh of antibiotics, macroleads and aminoglycosides therefore they can influence bacteria at which resistance to them is observed. The organisms resistant to above-mentioned dosage forms can be sensitive to a moksifloksatsin.
Moxifloxacin is active concerning the majority of strains of the following microorganisms
Gram-positive bacteria:
Corynebacterium diphtheriae Microbacterium spp.
Micrococcus luteus [including the strains resistant to erythromycin, gentamycin, tetracycline and/or Trimethoprimum]
Staphylococcus aureus [including strains, steady to metitsilinu, to erythromycin, gentamycin, ofloksatsinu, to tetracycline and/or Trimethoprimum]
Staphylococcus epidermidis [including strains, steady to metitsilinu, to erythromycin, gentamycin, ofloksatsinu, to tetracycline and/or Trimethoprimum]
Staphylococcus heamolyticus [including strains, steady to metitsilinu, to erythromycin, gentamycin, ofloksatsinu, to tetracycline and/or Trimethoprimum]
Staphylococcus hominis [including strains, steady to metitsilinu, to erythromycin, gentamycin, ofloksatsinu, to tetracycline and/or Trimethoprimum]
Staphylococcus warneri [including the strains resistant to erythromycin]
Streptococcus mitis [including the strains resistant to penicillin, erythromycin, tetracycline and/or Trimethoprimum]
Streptococcus pneumoniae [including the strains resistant to penicillin, erythromycin, gentamycin, tetracycline and/or Trimethoprimum]
Streptococcus viridans [including the strains resistant to penicillin, erythromycin, tetracycline and/or Trimethoprimum]
Gram-negative bacteria:
Acinetobacter spp.
Haemophilus alconae Haemophilus influenzae [including the strains resistant to ampicillin]
Klebsiella pneumoniae [including the strains resistant to ampicillin]
Moraxella catarrhalis Pseudomonas aeruginosa
Other microorganisms:
Chlamydia trachomatis
Indications
– topical treatment of the bacterial conjunctivitis caused by microorganisms, sensitive to drug,
to Dig in the Route of administration and doses in the affected eye (eyes) on 1 drop 3 times a day within 4 days.
It is not necessary to touch with a pipette tip eyes or any other surface to avoid pollution of contents of a bottle.
Use of eye drops of Vigamoks for children and newborns, also effectively and safely, as well as when using drug at adults can be also appointed in the same dose as for adult patients.
Side effects
Local:
– misting of sight
– passing discomfort and eye pain
– an itching
– xerophthalmus
– a keratitis
– subconjunctival hemorrhage
System:
At absorption in rare instances can be noted
– vascular collapse, a Quincke’s disease (including a laryngeal edema, throats or faces)
– a loss of consciousness, a headache
– respiratory insufficiency, dispnoe, pharyngitis, rhinitis
– urticaria
– anaphylactoid reactions
of the Contraindication
– hypersensitivity to a moksifloksatsin, other hinolona or auxiliary components of drug
Medicinal interactions
Unlike other ftorokhinolon clinically significant medicinal interactions between moksifloksatsiny system use and itrakonazoly, theophylline, warfarin, digoxin, oral contraceptives, pro-benzidine, ranitidine or Glyburidum are noted. Moxifloxacin does not inhibit CYP3A4, CYP2D6, CYP2C9 or CYP1A2 that says that it, most likely, does not change pharmacokinetic properties of drugs, metaboliziruyemy P450 cytochrome isoenzymes.
Special instructions
As well as in a case with other antibacterial agents, prolonged use of drug can cause the overgrowth of insensitive organisms including mushrooms. At superinfection it is necessary to stop administration of drug and to consider alternative methods of therapy. With care to appoint along with the drugs extending QT interval as risk of developing cardiac arrhythmias increases.
Not to administer the drug subkonjyunktivalno or in an anterior chamber of an eye.
Contact lenses
in the presence of symptoms of bacterial conjunctivitis the carrying contact lenses before full treatment is not recommended to the patient.
Risk of development of anaphylactic reaction
At the patients using system hinolonovy drugs deadly reactions of hypersensitivity (anaphylaxis) sometimes right after reception of the first dose were observed heavy, in some cases. Some reactions were followed by collapse of a cardiovascular system, a loss of consciousness, vascular hypostasis (including guttural, pharyngeal or front swelled), obstruction of pneumatic ways, short wind, urticaria and an itching. If at use of a moksifloksatsin the allergic reaction develops, it is necessary to stop administration of drug. The heavy acute reaction of hypersensitivity on moxifloxacin or any other component of drug can demand the emergency therapy. According to clinical indications apply oxygen and carry out artificial respiration.
Pregnant women should appoint pregnancy of Vigamoks only if the expected advantage of its use considerably exceeds potential risk for a fruit.
A lactation
As it is unknown whether moxifloxacin gets into breast milk, it is necessary to appoint with care Vigamoks to nursing mothers.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
As well as in a case with other eye drops, after burying the temporary ambiguity of sight or other visual concerns is possible that it can negatively affect ability to drive the car or other potentially dangerous mechanisms. In this case it is necessary to wait some time before recovery of sight.
The overdose
the Limited capacity of a konyyuktivalny bag makes impossible overdose by the ophthalmologic drug Vigamoks.
Also intoxication after accidental intake is excluded.
A form of release and packing
Eye drops of 0.5% 5 ml, in bottles from translucent plastic with Drop-Tainer dropper doser. Each bottle together with the instruction for use in the state and Russian languages is packed into a cardboard box.
To Store storage conditions at a temperature from +20 to + 250C.
To store in the places inaccessible for children!
2 years
not to apply a period of storage after an expiration date.
An expiration date after the first opening – 4 weeks at a temperature from +20 to + 250C.
Prescription status
According to the prescription
Producer Alcon Laboratories, Inc., the UNITED STATES OF AMERICA
Fort Worth, TX 76134 USA
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods)
of Predstavitelstvo Alkon Pharmasyyutikalz Ltd.
Ph.: +7 (727) 256 02 05
Fax: +7 (727) 256 06 81
E-mail address:
To develop Tatyana.Yem@AlconLabs.com
Additional information
| Ingredient |
|---|















Reviews
There are no reviews yet.