Description
The instruction for medical use
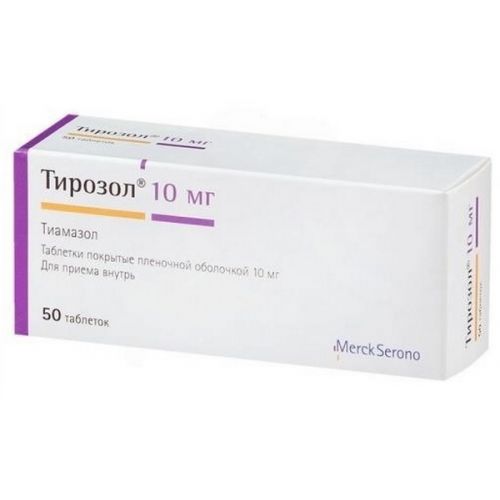
of Tirozol® medicine
the Trade name
of Tirozol®
the International unlicensed
name Thiamazolum (Thiamazole)
the Dosage form
of the Tablet, film coated 5 mg, 10 mg
Structure
of the Tablet, film coated 5 mg
active agent – Thiamazolum of 5 mg
excipients: silicon dioxide colloidal, sodium of starch is glikolit (type C), magnesium stearate, a gipromelloza 2910/15, talc, cellulose powdery, starch corn, lactoses monohydrate
structure of a film cover: ferrous oxide yellow (E 172), dimetikon 100, a macrogoal 400, the titan dioxide (E 171), a gipromelloza of 2910/15
of the Tablet, film coated 10 mg
active agent – Thiamazolum of 10 mg
excipients: silicon dioxide colloidal, sodium of starch is glikolit (type C), magnesium stearate, a gipromelloza 2910/15, talc, cellulose powdery, starch corn, lactoses monohydrate
structure of a film cover: ferrous oxide yellow (E 172), ferrous oxide red (E 172), dimetikon 100, a macrogoal 400, the titan dioxide (E 171), a gipromelloza of 2910/15
the Description
Round biconvex tablets, coated light yellow color with a notch for a break on both parties of a tablet, diameter – about 9.1 mm, thickness – about 3.7 mm (for a dosage of 5 mg),
Round biconvex tablets, coated gray-orange color with a notch for a break on both parties of a tablet, diameter – about 9.1 mm, thickness – about 3.7 mm (for a dosage of 10 mg).
Pharmacotherapeutic group
Drugs for treatment of diseases of a thyroid gland. Anti-thyroid drugs. Sulfur-containing derivatives of an imidazole. Thiamazolum.
The ATX H03BB02 code
the Pharmacological
Pharmacokinetics Thiamazolum properties at intake quickly and almost is completely soaked up. The maximum concentration in plasma is reached within 0.4 – 1.2 hours. Practically does not contact proteins of blood plasma. Thiamazolum collects in a thyroid gland where it is slowly metabolized. Despite changes of serumal concentration, accumulation of Thiamazolum in a thyroid gland nevertheless results in steady concentration. It leads to long action, about 24 hours after use of a single dose. The dependence of kinetics on a functional condition of a thyroid gland is not revealed. Elimination half-life makes about 3 – 6 hours, in a liver failure it increases. Metabolism of Thiamazolum is carried out in kidneys and a liver, low excretion with a stake is observed that indicates enterogepatichesky circulation. 70% of substance are removed through kidneys within 24 hours. Only a small amount is removed in an invariable look. There are no data on pharmacological activity of metabolites now.
The pharmacodynamics
of Tirozol® inhibits in a dose-dependent way inclusion of iodine in tyrosine and, thereby, neosynthesis of hormones of a thyroid gland. This property allows to carry out symptomatic therapy of a hyperthyroidism irrespective of its reason. Now there are no exact data on a further possibility of Thiamazolum to have effect on ‘natural course’ immunological of the caused hyperthyroidism (Graves disease) i.e. whether it can suppress the main immunopathogenic process. It does not influence discharge of earlier synthesized hormones from a thyroid gland. It explains why in various cases various duration of stage of latency until achievement of normalization of serumal concentration of thyroxine and triiodothyronine, and, thus, before clinical improvement is observed. Influence on the hyperthyroidism which is result of discharge of hormones after destruction of cells of a thyroid gland does not appear, for example, after therapy by radioiodine or in a thyroiditis.
Indications
Treatment of a hyperthyroidism, including the following:
– conservative treatment of a hyperthyroidism, especially in the small or absent craw,
– preparation for surgery at all forms of a hyperthyroidism,
– preparation for the planned treatment by radioiodine, in particular at patients with severe forms of a hyperthyroidism,
– periodic therapy after treatment by radioiodine.
– preventive treatment at patients with the latent (latent) hyperthyroidism, autonomous adenomas or with a hyperthyroidism in the anamnesis for which treatment by iodine is obligatory (for example, at inspection with use of iodinated contrast environments).
The route of administration and doses
the Dosage at adults
Depending on disease severity and consumption of iodine, treatment usually begins with daily doses of Tirozola® from 10 to 40 mg. In many cases, suppression of production of hormones of a thyroid gland can usually be reached at use of initial doses of 20 – 30 mg of Tirozola® a day. In less hard cases, use of the full blocking dose can not be required, and it is possible to consider the possibility of use of lower initial dose. In hard cases of development of a hyperthyroidism the initial dose of 40 mg of Tirozola® can be required.
The dose is adjusted individually depending on a condition of a metabolism of the patient – thus as it will be shown by development of the status of hormones of a thyroid gland.
For maintenance therapy one of the following options of treatment is recommended:
a) The daily maintenance dose makes 5 – 20 mg of Tirozola® in a combination with left thyroxine for avoidance of a hypothyroidism.
b) Monotherapy in daily doses of 2.5 – 10 mg of Tirozola®.
At the hyperthyroidism caused by iodine higher dosages can be required.
A dosage at children
Use for children and teenagers (from 3 to 17 years)
the Initial dose of treatment of children and teenagers (from 3 to 17 years) has to be it is calculated with calculation of body weight of patients. As a rule, treatment is begun with the dose 0.5 mg/kg divided into two or three equal parts. For maintenance therapy, the daily dose can be reduced and given depending on reaction of the patient to treatment once a day. Additional treatment by left thyroxine can be required for prevention of a hypothyroidism.
The general daily dose of Tirozol should not exceed 40 mg/days.
Conservative treatment of a hyperthyroidism
the Purpose of therapy consists in achievement of an euthyroid condition of a metabolism and long-term remission after the treatment limited on duration. Depending on the individual patients receiving treatment, remission can be reached at at most 50% of patients after one year. Frequency of remission varied in considerable limits. The probable factors having the impact are hyperthyroidism type (immunogene or not immunogene), the treatment duration, Thiamazolum dose and also intake of iodine with food or its iatrogenic receipt.
At conservative treatment of a hyperthyroidism the therapy usually continues during from 6 months to 2 years (on average – 1 year). From the statistical point of view, the probability of remission increases together with increase in duration of therapy.
In cases of impossibility of achievement of remission of a disease, certain therapeutic measures, Tirozol® it is possible to apply in the form of long-term anti-thyroid therapy in lower dosage without addition or a combination with low doses of left thyroxine.
Patients with the increased craw and a tracheostenosis have to undergo, if necessary, only short-term treatment of Tirozolom® as its long-term use can lead to growth of a craw. Attentive observation of therapy (can be required (content of TSH (thyroid stimulating hormone), a trachea gleam). It is preferable to carry out treatment to combinations with additional use of hormones of a thyroid gland.
Preoperative therapy
Preliminary treatment can be performed to achievement of an euthyroid condition of a metabolism for the purpose of risk reduction, connected with surgery. Depending on individual requirement duration of treatment can be about 3 – 4 weeks or longer.
Surgery has to be performed at once as the patient will reach an euthyroid state as, otherwise, replenishment of hormones of a thyroid gland can be required. Treatment can be finished in one day prior to surgery.
Тирозол® increases risk of damage of tissue of thyroid gland and bleeding from it that can be compensated by addition in preoperative therapy of high doses of iodine within ten days before operation (Plummer yodoterapiya).
Treatment before therapy by radioiodine
Achievement of a condition of an euthyroid metabolism before therapy by radioiodine is an important factor, in particular, in a heavy hyperthyroidism as post-therapeutic thyrocardiac crisis after such therapy without performing preliminary treatment was in some cases observed.
Note: Derivatives of a tionamid can reduce radio sensitivity of tissue of thyroid gland. At the planned therapy by radioiodine, in autonomous adenoma it is necessary to avoid activation of paranodulyarny fabric by means of preliminary treatment.
Periodic anti-thyroid therapy after treatment by radioiodine
Duration of treatment and doses which are subject to use need to be determined individually depending on weight of a clinical picture of an approximate span prior to manifestation of efficiency of therapy by radioiodine (about 4-6 months).
Preventive treatment at patients with risk of developing a hyperthyroidism as a result of use of iodinated substances in the diagnostic purposes
generally, daily doses of 10 – 20 mg of Thiamazolum and/or 1 g of perchlorate within about 10 days are applied (for example, to the contrast environments removed through kidneys). Duration of treatment depends on a span during which iodinated substance is in an organism.
Special groups of patients
At patients with a liver failure the lowered plasma clearance of Thiamazolum is observed. Therefore it is necessary to apply lower dose of drug, and for patients it is necessary to conduct attentive observation. Owing to insufficient amount of pharmacokinetic data on use of Tirozola® for patients with a renal failure, it is necessary to carry out correction of individual dosing with care, and it is recommended to conduct attentive observation. The dose has to be lower. Though there is no accumulation of drug at patients of advanced age, nevertheless it is necessary to carry out correction of individual dosing with care, and it is recommended to conduct attentive observation.
The route of administration
of the Tablet needs to be swallowed entirely, washing down with enough liquid.
During initial therapy with use of high doses concerning a hyperthyroidism, the above-stated single doses can be divided into several receptions and to accept them during the day through equal intervals of time.
The maintenance dose can be accepted for 1 time in the morning – in time or after a breakfast.
Assessment of side effects bases side effects on the following frequency classification:
Very frequent: ≥ 1/10
Frequent: ≥ 1/100, & lt, 1/10
Infrequent: ≥ 1/1000, & lt, 1/100
Rare: ≥ 1/10000, & lt, 1/1000
Very rare: & lt, 1/10000
Blood and lymphatic systems
Infrequently: the agranulocytosis was observed in 0.3% – 0.6% cases. Symptoms can appear even weeks and months later after an initiation of treatment and to result in need of drug withdrawal,
Is very rare: generalized lymphadenopathy, thrombocytopenia, pancytopenia.
Endocrine system
Very seldom: an insulin autoimmune syndrome with a hypoglycemia (with the significant decrease in content of glucose in blood).
Nervous system
Seldom: reversible change of flavoring feelings,
Is very rare: neuritis, polyneuropathy.
Gastrointestinal disturbances
Very seldom: increase in sialadens, vomiting.
Disturbances from a liver and biliary tract
It is very rare: cholestatic jaundice and toxic hepatitis. Symptoms usually passed after drug withdrawal. The differential diagnosis between clinically not expressed symptoms of a cholestasia during treatment and the disturbances caused by a hyperthyroidism – such as increases in GGT (gamma glutamiltransferazy) and alkaline phosphatase or its isoenzyme specific to
Disturbance bones has to be carried out from skin and hypodermic fabrics
Very often: allergic skin reactions (itching, reddening, rashes). They usually have the easy form of weight and often take place at therapy continuation,
Is very rare: generalized enanthesis, hair loss, volchanochnopodobny syndrome.
Disturbances from musculoskeletal and connective tissue
it is frequent: gradual development of an arthralgia after several months of the carried-out therapy
of the Complication of the general character and reaction in the injection site
is rare: temperature increase, weakness, increase in body weight.
Contraindications
– hypersensitivity to Thiamazolum, other derivatives of a tionamid or to any of the excipients which are a part of drug.
– an agranulocytosis, a granulocytopenia,
– a cholestasia before an initiation of treatment,
– earlier noted injuries of marrow after treatment by Thiamazolum or carbimazole,
– combination therapy by Thiamazolum and hormones of a thyroid gland during pregnancy and a lactation
– children’s age up to 3 years
With care
– a craw of the big sizes with a tracheostenosis
Medicinal interactions
the Iodine deficiency increases reaction of a thyroid gland to Thiamazolum and vice versa the increased content of iodine reduces this reaction. Other types of direct interaction with other medicines are unknown. However it is necessary to consider that metabolism and elimination of other medicines can accelerate in a hyperthyroidism. They are normalized at achievement of normalization of function of a thyroid gland. If it is necessary, it is necessary to adjust a dosage.
Moreover, emergence of the signs indicating improvement of a condition of a hyperthyroidism can mean normalization of superactivity of anticoagulants at patients with a hyperthyroidism.
The special
instructions Tirozol® are not recommended to be applied at patients with hypersensitivity reactions in the anamnesis (for example, allergic rash, an itching).
Тирозол® it is necessary to apply only in the form of short-term therapy and on condition of attentive observation of patients with the increased craw and risk of a tracheostenosis owing to growth of a craw.
The agranulocytosis was noted in about 0.3 – 0.6% cases. Therefore before therapy of patients it is necessary to inform on the accompanying symptoms (stomatitis, pharyngitis, fever). It usually develops in the first weeks of treatment, but also can be shown also in several months after the beginning of therapy and also when resuming therapy. It is recommended to conduct attentive observation of indicators of blood tests till the beginning of therapy, especially at patients with the preexisting granulocytopenia. In case of any of these symptoms, especially in the first weeks of treatment, patients need to recommend to report about it at once to the attending physician for purpose of blood test. In case of confirmation of an agranulocytosis, use of medicine should be suspended. At use of drug in the range of the recommended doses also other myelotoxic side effects occasionally were noted. They were often observed at use of very high doses of Thiamazolum (about 120 mg a day). These dosages should be reconsidered taking into account special indications (heavy course of a disease, thyrocardiac crisis). At development of toxicity for marrow during treatment by Thiamazolum it is necessary to suspend use of this drug and if it is necessary, to pass to use of the anti-thyroid drug relating to other group of medicines. High dosages can lead to a subclinical or clinical hypothyroidism and growth of a craw owing to increase in content of TSH. Therefore the dose of Thiamazolum needs to be lowered at once on later achievements of an euthyroid condition of a metabolism, and if it is necessary, it is necessary to appoint left thyroxine in addition. It is not necessary to stop completely use of Thiamazolum and to continue treatment only by one left thyroxine. Growth of a craw in time therapy by Thiamazolum, despite suppression of production of TSH, is result of a basic disease, and this effect cannot be prevented additional treatment by left thyroxine. Achievement of normal content of TSH is crucial for minimization of risk of development or deterioration in an endocrine orbitopatiya. However this state often does not depend on course of a disease of a thyroid gland. Such complication in itself is not the reason for modification of the sufficient mode of treatment, and it cannot be considered as adverse reaction to the carried-out appropriate therapy. In rare instances the hypothyroidism with late manifestation after anti-thyroid therapy without any additional ablative measures can develop. It probably represents the adverse reaction connected with drug, but, at the same time, is considered as inflammatory and destructive process in a parenchyma of a thyroid gland owing to a basic disease. Decrease patholologically in the increased consumption of energy in a hyperthyroidism can lead to possible increase in body weight during treatment by Thiamazolum. Patients need to be informed on what at consumption of energy will normalizovyvatsya together with improvement of the general state. Tirozol contains lactose therefore this drug cannot be used at the patients having the rare hereditary disorders connected with intolerance of a galactose, either deficiency of lactase, or glucose galactose absorption disturbance.
Pregnancy and the period of a lactation
generally, pregnancy has positive effect on a hyperthyroidism. Nevertheless, the provodeniye nebkhodimost treatment of a hyperthyroidism, especially, in the first months of pregnancy is possible. Not cured hyperthyroidism during pregnancy can lead to serious complications, such as premature births and malformations. However, the hypothyroidism caused by treatment by improper doses of Thiamazolum can be also associated with an abortion.
Тирозол® gets through a placental barrier and in blood of a fruit can reach concentration, equal subjects which are observed in mother’s serum. Use of improper dosages of drug can lead to formation of a craw and a hypothyroidism at a fruit and also to decrease in body weight of the newborn at the birth. Cases of a partial aplasia of skin at the newborns born from mothers receiving Thiamazolum were repeatedly noted. This defect recovers spontaneously within several weeks.
In addition to it, a certain picture of various malformations is associated with therapy by high doses of Thiamazolum in the first weeks of pregnancy – for example, a hoanalatreziya, a gullet atresia, a hypoplasia of nipples, a delay of intellectual and also motive development. Contrary to it, several researches of separate cases of prenatal influence of Thiamazolum did not reveal neither any morphological disorders of development, nor impact on development of a thyroid gland, or physical and intellectual development of children. As it is impossible to exclude completely embriotoksichesky action, Tirozol® can be applied during pregnancy only after careful assessment of advantage and risk and only in the lowest effective dose without additional use of hormones of a thyroid gland.
Тирозол® gets into breast milk in which it can reach the concentration corresponding to concentration in serum at mothers therefore there is a risk of development of a hypothyroidism at babies.
It is possible to carry out chest feeding during treatment by Thiamazolum, however, in this case, it is possible to use only low doses – to 10 mg a day and without additional use of hormones of a thyroid gland.
It is necessary to carry out regular monitoring of function of a thyroid gland at babies.
Features of influence of medicine on sposobnostyupravlyat the vehicle or potentially dangerous mechanisms
Data are absent. It is necessary to consider a possibility of development of side effects (a headache, dizziness, weakness, etc.).
Overdose
Symptoms: the overdose leads to a hypothyroidism with symptoms of the lowered metabolism. By means of feedback effect, there is an activation of a front share of a hypophysis to the subsequent growth of a craw.
Treatment: a dose decline as soon as possible after achievement of an euthyroid condition of a metabolism and if it is necessary, addition in left thyroxine use therapy.
Negative consequences of accidental use of high doses of Thiamazolum are unknown.
A release form
On 25 tablets in blister strip packagings from PVC of a film and aluminum foil.
On the 2nd blister strip packagings together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions in the dry place at a temperature not above 25ºC.
To store out of children’s reach!
A period of storage
4 years
not to use drug after expiry date
Prescription status
According to the prescription
the Producer
Merk of KGaA, Darmstadt, Germany
the Name and the country of the owner of the registration certificate:
Merk of Serono GmbH, Germany
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods): Representative office ‘Takeda Osteuropa Holding GmbH” (Austria) in Kazakhstaneg. Almaty, Begalin St., 136anomer of phone number (727) 2444004 Fax number (727) 2444005 E-mail address:
To Develop DSO-KZ@takeda.com
Additional information
| Ingredient |
|---|


















Reviews
There are no reviews yet.