Description
The instruction for use
medicine for experts
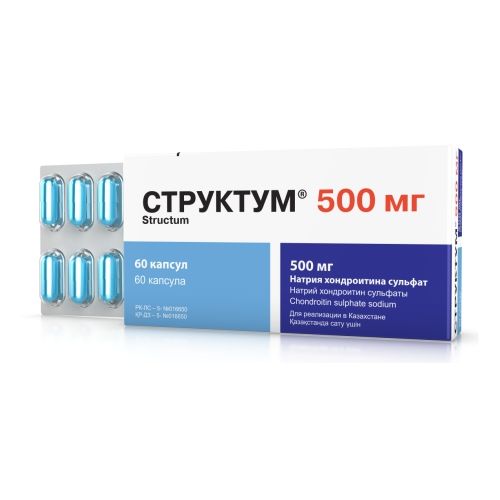
Struktum ®
A trade name
Struktum ®
the International unlicensed
name Chondroitin sodium sulfate
the Dosage form
of the Capsule of 250 mg and 500 mg
Structure
One capsule contains
active agent – chondroitin sodium sulfate 250 mg and 500 mg,
excipients: talc, gelatin, titan dioxide, indigotin.
Description
Gelatin capsules of blue color, flavourless.
Pharmacotherapeutic group
Other means for treatment of diseases of a musculoskeletal system.
The code of automatic telephone exchange M09AX02
the Pharmacological
Pharmacokinetics Later properties of intake chondroitin sulfate is quickly absorbed from digestive tract. The maximum concentration in blood plasma is reached depending on the initial content of chondroitin of sulfate
in blood, on average in 3-4 hours after administration of drug. The bioavailability chondroitin of sulfate is 13%. Accumulation chondroitin of sulfate is carried out mainly in synovial fluid of joints. Removal is carried out generally by kidneys during 24 h.
The pharmacodynamics
Struktum influences exchange processes in a hyaline cartilage, reduces degenerative changes in cartilaginous tissue of joints, stimulates biosynthesis of glucosaminoglycans. Struktum possesses the prolonged action and reduces number of exacerbations of an osteoarthrosis. Has analgeziruyushchy and anti-inflammatory effect, reduces pains at rest and when walking. At the Struktum drug treatment the morbidity decreases, the mobility of the affected joints improves, the need for non-steroidal anti-inflammatory drugs decreases.
Indications
– degenerative diseases of joints and a backbone: osteoarthroses, intervertebral osteochondrosis.
The route of administration and doses
Struktum accept inside, washing down with water.
The adult appoint 1000 mg a day – on 2 capsules of 250 mg or on 1 capsule of 500 mg 2 times a day.
The recommended duration of an initial course of treatment is 6 months, the drug after-effect period after its cancellation – 3-5 months depending on localization and a stage of a disease, duration of repeated courses of treatment is established individually and depends on localization and degree of arthrosis and osteochondrosis.
Side effects
– allergic reactions
– nausea, pains in epigastriums, a meteorism, diarrhea, zaporoshok
Contraindications
– individual intolerance of drug
– tendency to bleeding, thrombophlebitises
– phenylketonuria
– pregnancy and a lactation
– children’s age up to 12 years.
Medicinal interactions
At simultaneous use of Struktum with other medicines strengthening of effect of indirect anticoagulants, antiagregant, fibrinolitik is possible.
Special indications
of Feature of influence on ability to run transport and potentsialnoopasny mechanisms
Use of drug does not affect ability to control of transport and potentially dangerous mechanisms
With care it is necessary to appoint to patients with tendency to bleedings.
Overdose
Symptoms: strengthening of side effects.
Treatment: gastric lavage, symptomatic therapy.
The form of release and packing
of the Capsule of 250 mg are packed into blisters on 15 pieces, in a cardboard pack 4 blisters (60 capsules) with the instruction for use.
Capsules on 500 mg are packed into blisters on 12 pieces, in a cardboard pack of 5 blisters, or on 15 pieces, in a cardboard pack 4 blisters, or on 20 pieces, in a cardboard pack 3 blisters (60 capsules) with the instruction for use.
To Store storage conditions at
a temperature not higher than 250 S. Hranit out of children’s reach!
A period of storage
3 years – capsules on 250 mg, on 500 mg
not to use drug after an expiration date
Prescription status
According to the prescription
Pierre Fabr Medikament Production Producer, France
Progipharm, 45500 Zhiyen.
Additional information
| Ingredient |
|---|














Reviews
There are no reviews yet.