Description
The instruction for use
of medicine for experts
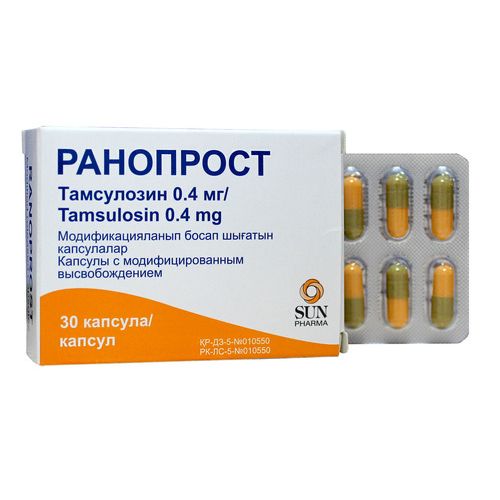
of Ranoprost
the Trade name
of Ranoprost
the International unlicensed
name Tamsulozin Lekarstvennaya
the Capsule form with the modified release
Structure
One capsule contains
active agent – a tamsulozin a hydrochloride of 0.4 mg,
excipients: microcrystalline cellulose, magnesium stearate, methacrylic acid – ethyl acrylate copolymer (1:1) dispersions (30%), methacrylic acid – the copolymer of ethyl acrylate (1:1), sodium hydroxide, triacetin, talc purified, titanium dioxide,
structure of the capsule: gelatin, solar yellow, quinolinic yellow, ferrous oxide yellow, ponso 4P, diamond blue, crimson, the titan the dioxide, coal activated, shellac.
The description
the Capsule 2 of No. in size, a lid of brown color with the inscription ‘R’, the body of orange color with the inscription ‘TSN 400’ black printing paint. Capsule contents – white or almost white powder.
Pharmacotherapeutic group
Drugs for treatment of a benign hyperplasia of a prostate. Alpha adrenoblocker.
The code of automatic telephone exchange G04CA02
the Pharmacological
Pharmacokinetics Later properties of intake tamsulozin is quickly and almost completely soaked up from digestive tract. Administration of drug after a meal affects absorbability of a tamsulozin. The uniformity of absorption can be settled by the patient if always to accept tamsulozin in one and too time after a meal. Tamsulozin shows linear kinetics.
After reception of a single dose of a tamsulozin after a meal, peak concentration in plasma is reached in 6 hours, at a steady state which is reached for the 5th day at a repeated dose, Cmax is about two thirds higher, than Cmax after reception of a single dose. There is a considerable fluctuation of level of concentration in plasma after reception of a single dose and repeated dosed
At men tamsulozin for 99% distribution volume very low contacts proteins of plasma, and (about 0.2 l/kg).
Tamsulozin is slowly metabolized in a liver with formation pharmacological of the active metabolites keeping high selectivity to α1А-адренорецепторам. The most part of active agent is present at blood in not changed look.
Regulation of a dose in a liver failure is not required.
Any of metabolites of drug does not show bigger activity, than an original component.
Tamsulozin and his metabolites, mainly, is brought with urine, about 9% of a dose are removed in not changed look. After reception of a single dose on a full stomach and on an empty stomach, elimination half-life of drug makes respectively 10 and 13 hours.
The pharmacodynamics
Selectively and competitively blocks postsynaptic α1А-адренорецепторы, being in smooth muscles of a prostate, a neck of a bladder and a prostatic part of an urethra. Reduces a tone of smooth muscles of a prostate, a neck of a bladder and a prostatic part of an urethra, improving urine outflow.
At the same time the symptoms of obstruction and irritation connected with a benign hyperplasia of a prostate decrease. Therapeutic it effektproyavlyatsya approximately in 2 weeks from an initiation of treatment. The ability to block α1В-adrenoceptors of unstriated muscles of vessels is much less significant at a tamsulozin therefore action on system the ABP is insignificant.
Indications
– the benign hyperplasia of a prostate (BHP).
A route of administration and doses
the Recommended daily dose – on one Ranoprosta capsule of 0.4 mg after a breakfast, washing down with enough water.
Capsules are recommended to be swallowed entirely, without chewing as it can influence the modified release of active component.
Side effects
– dizziness
– disturbance of an ejaculation
– nausea, vomiting, diarrhea, constipations
– drowsiness
– dryness in a mouth or swelled
Seldom
– a headache, an asthenia
– orthostatic hypotonia, heartbeat
– rhinitis
– reaction of hypersensitivity (itching and urticaria)
In some cases
– a loss of consciousness, a Quincke’s disease and a priapism
of the Contraindication
– hypersensitivity to Tamsulozin to a hydrochloride or any other component of drug
– orthostatic hypotension in the anamnesis
– the liver failure
Medicinal interactions
At a concomitant use Cimetidinum increases concentration in plasma, and furosemide – reduces, but as levels remain within admissible range, the dosage of drug does not change.
Diclofenac and warfarin increase the clearance rate of a tamsulozin a little.
There is a risk of strengthening of hypotensive effect at a concomitant use of the drugs lowering blood pressure including anesthetics and others α1-адреноблокаторы.
Special instructions
When using others α1-адреноблокаторов, at drug treatment the lowering of arterial pressure which can sometimes lead to an unconscious state in some cases can be observed. At the first symptoms of orthostatic hypotension (dizziness, weakness) the patient has to sit down or lay down and remain in this situation until signs do not disappear. Before beginning therapy with drug, the patient has to be examined to exclude presence of other diseases which can cause the same symptoms, as well as a benign hyperplasia of a prostate. Before an initiation of treatment and regularly during therapy the manual rectal inspection and if it is required, definition of specific antigen of a prostate (PSA) has to be carried out.
Drug it is necessary to appoint with care the patient with a heavy renal failure (clearance of creatinine less than 10 ml/min.) as this group of patients was not studied.
Tamsulozin is intended only for men.
Drug is not used in pediatric practice.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
of Data on influence of a tamsulozin on ability of control of motor transport or work with mechanisms it is not provided. However, the patients taking the drug should consider a possibility of manifestation of the following side effects: drowsiness, dizziness and syncope.
Overdose
Symptoms: acute hypotonia
Treatment: symptomatic therapy for a cardiovascular system. It is necessary to control renal function and to provide maintenance therapy. Removal of drug by dialysis does not result in desirable result as linking of a tamsulozin with proteins of plasma extremely high. For prevention of further absorption of drug the gastric lavage, intake of activated carbon or osmotic laxative, for example sulfate magnesium is reasonable.
A form of release and packing
of the Capsule with the modified release No. 30. Each capsule supports Tamsulozin a hydrochloride of 0.4 mg
PVdC (polyvinylidene chloride) / PVC Blisters (polyvinylchloride).
To Store storage conditions at a temperature not above +25 °C, in the place protected from light and moisture.
To store out of children’s reach!
An expiration date
2 years
not to use drug after an expiration date.
Prescription status
According to the prescription
Producers Ranbaksi Laboratories Limited.
Іndustrial Area – 3, Dewas, M.P. – 455,001, India
Additional information
| Ingredient |
|---|
















Reviews
There are no reviews yet.