Qlaira® (Estradiol Valerate/Dienogest) 28 film-coated tablets
$40.00
Description
Other brand names: NATAZIA® (Estradiol Valerate/Dienogest), VISANNE® (Dienogest)
Composition
For one dark yellow film-coated tablet:
Active ingredient:
estradiol valerate micro 20 – 3,000 mg
Auxiliary components:
lactose monohydrate,
corn starch,
pregelatinized corn starch,
povidone-25,
magnesium stearate
Sheath:
hypromellose,
macrogol-6000,
talc,
titanium dioxide
dye iron oxide yellow.
For one pink film-coated tablet:
Active ingredients :
Estradiol valerate micro 20 – 2,000 mg
Dienogest micro – 2,000 mg,
Auxiliary components:
lactose monohydrate,
corn starch,
pregelatinized corn starch,
povidone-25,
magnesium stearate
Shell:
hypromellose,
macrogol-6000,
talc,
titanium dioxide
iron dye oxide red.
For one pale yellow film-coated tablet:
Active ingredients:
estradiol valerate micro 20 – 2,000 mg
Dienogest micro – 3,000 mg
Auxiliary components:
lactose monohydrate,
corn starch,
pregelatinized corn starch,
povidone-25,
magnesium stearate
Sheath:
hypromellose,
macrogol-6000,
talc,
titanium dioxide
dye iron oxide yellow.
For one red film-coated tablet:
Active ingredient:
estradiol valerate micro 20 – 1,000 mg
Auxiliary components :
lactose monohydrate,
corn starch,
pregelatinized corn starch,
povidone-25,
magnesium stearate
Sheath:
hypromellose,
macrogol-6000,
talc,
titanium dioxide
iron dye oxide red.
For one white film-coated tablet (placebo):
Auxiliary components:
lactose monohydrate,
corn starch,
povidone-25,
magnesium stearate
Sheath:
hypromellose,
talc,
titanium dioxide.
Pharmachologic effect
The contraceptive effect of combined oral contraceptives (COCs) is based on the interaction of various factors, the most important of which are suppression of ovulation and changes in the properties of cervical mucus. Along with the prevention of unwanted pregnancy, COCs have a number of positive properties, which, taking into account also the negative properties, can help in choosing the most appropriate method of contraception.
In women taking COCs, the soreness and intensity of menstrual bleeding decreases, as a result of which the risk of iron deficiency anemia decreases. In addition, there is evidence of a reduced risk of endometrial and ovarian cancer.
The estrogen in Qlaira’s preparation is estradiol valerate, the precursor of natural human 17β-estradiol (1 mg estradiol valerate corresponds to 0.76 mg of 17β-estradiol) The estrogen component used in this COC is thus different from the estrogens commonly used in COCs, which are synthetic estrogens ethinyl estradiol or its precursor mestranol, both containing an ethynyl group at position 17α. This group is responsible for a higher metabolic stability, but also a more pronounced effect on the liver.
Taking the drug Qlaira leads to a less pronounced effect on the liver compared to three-phase COCs containing ethinylestradiol and levonorgestrel. It has been shown that the effect on the concentration of sex steroid binding globulin (SHBG) and hemostasis parameters is less pronounced.
In combination with dienogest estradiol valerate shows an increase in high density lipoprotein (HDL), while the concentration of low density lipoprotein cholesterol (LDL) decreases slightly.
Dienogest is an oral progestogen with additional partial antiandrogenic effects. Its estrogenic, antiestrogenic and androgenic properties are negligible. Due to the special chemical structure, a spectrum of pharmacological action is provided that combines the most important advantages of 19-nor-progestogens and progesterone derivatives.
Indications
Oral contraception.
Application during pregnancy and lactation
Taking the drug Qlaira is contraindicated during pregnancy and lactation.
If pregnancy is detected or suspected while taking Qlaira, the drug should be immediately discontinued and a doctor should be consulted.
However, extensive epidemiological studies have not revealed any increased risk of developmental defects in children born to women who received sex hormones (including COCs) before pregnancy or teratogenic effects when sex hormones were taken inadvertently in early pregnancy.
If you are pregnant or breastfeeding, consult your healthcare professional before taking any medication.
Contraindications
Qlaira® should not be used in the presence of any of the conditions listed below. The drug should be stopped immediately if any of these conditions develop for the first time while taking it:
- hypersensitivity to active substances or any of the excipients;
- thrombosis (venous and arterial) and thromboembolism currently or in history (including deep vein thrombosis (DVT), pulmonary embolism (PE), myocardial infarction (MI), current or history of stroke);
- conditions preceding thrombosis (including transient ischemic attacks, angina pectoris) at present or in history;
- identified acquired or hereditary predisposition to venous or arterial thrombosis, including resistance to activated protein C, antithrombin III deficiency, protein C deficiency, protein S deficiency, hyperhomocystsinemia, antibodies to phospholipids (antibodies to cardiolipin, lupus anticoagulant);
- the presence of a high risk of venous or arterial thrombosis (see “Special instructions”);
- migraine with focal neurological symptoms, incl. history;
- diabetes mellitus with vascular complications;
- pancreatitis with severe hypertriglyceridemia at present or in history;
- liver failure and severe liver disease (before normalization of liver function indicators);
- liver tumors (benign and malignant), currently or in history;
- identified hormone-dependent malignant tumors (including genitals or mammary glands) or suspicion of them;
- vaginal bleeding of unknown origin;
- pregnancy or suspicion of it;
- breastfeeding period;
- lactose intolerance, lactase deficiency, glucose-galactose malabsorption.
WITH CAUTION
- If any of the diseases / conditions / risk factors listed below are currently available, then the potential risk and the expected benefit of using the Qlaira® drug in each individual case should be carefully correlated:
- risk factors for the development of thrombosis and thromboembolism (smoking; obesity; dyslipoproteinemia; arterial hypertension; migraine; heart valve disease; heart rhythm disturbances; extensive surgical interventions without prolonged immobilization);
- other diseases in which peripheral circulation disorders may occur (diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, Crohn’s disease and ulcerative colitis, sickle cell anemia);
- hereditary angioedema;
- hypertriglyceridemia;
- diseases that first appeared or worsened during pregnancy or against the background of previous use of sex hormones (for example, cholestatic jaundice, cholestatic pruritus, cholelithiasis, otosclerosis with hearing impairment, porphyria, herpes of pregnant women, Sydenham’s chorea);
- postpartum period.
Side effects
System organ class Often Uncommon Rarely
Infections and invasions Fungal infection, vaginal candidiasis, vaginal infection, unspecified
Metabolism and alimentary disorders
Increased appetite, fluid retention, hypertriglyceridemia
Nervous system
Headache (including tension headache), Depression / decreased mood, decreased libido, mental disorder, mood changes, dizziness, affective lability, aggressiveness, anxiety, dysphoria, increased libido, nervousness, anxiety, sleep disturbance, stress, attention deficit, paresthesia, vertigo
Organ of vision
Contact lens intolerance, dryness of the mucous membrane of the eyes, swelling of the eyelids
The cardiovascular system
Increased blood pressure, migraine (including with and without aura), Bleeding from varicose veins, hot flashes to the face, decreased blood pressure, pain along the veins
From the gastrointestinal tract
Abdominal pain (including bloating), Diarrhea, nausea, vomiting Constipation, dyspepsia, gastroesophageal reflux
From the liver and biliary tract
Increased ALT activity, focal nodular hyperplasia of the liver
On the part of the skin and subcutaneous tissues
AcneAlopecia, pruritus (including generalized itching and itchy rash), rash (including spotted rash), hyperhidrosis
Allergic skin reaction, including allergic dermatitis and urticaria, chloasma, dermatitis, hirsutism, hypertrichosis, neurodermatitis, pigmentation disorders, seborrhea, unspecified skin lesions
From the musculoskeletal and connective tissue
Back pain, muscle cramps, feeling of heaviness
On the part of the genitals and mammary gland
Lack of menstrual bleeding, discomfort in the mammary glands, pain in the mammary glands, nipple soreness, nipple pain, painful menstrual bleeding, irregular menstrual bleeding (metrorrhagia) Breast enlargement, diffuse breast lump, dysplasia of the cervical epithelium, dysfunctional bleeding, dysfunctional , fibrocystic breast disease, menorrhagia, cysts in the ovaries, pain in the pelvic region, premenstrual syndrome, uterine leiomyoma, uterine spasms, vaginal discharge, dryness in the vulvovaginal region, Benign neoplasm in the mammary gland, breast cyst, bleeding during intercourse , galactorrhea, vaginal bleeding, hypomenorrhea, delayed menstrual bleeding, ruptured ovarian cyst, burning sensation in the vagina, uterine / vaginal bleeding (including spotting, vaginal odor, vulvovaginal discomfort)
On the part of the blood and lymphatic system
Lymphadenopathy
Common Symptoms
Weight gain Irritability, edema, weight loss Chest pain, fatigue, malaise
The following serious adverse events have been reported in women using COCs (which include Qlaira®):
arterial and venous thrombosis and thromboembolic complications; worsening of the course or provoking symptoms of angioedema; liver tumors; acute pancreatitis
Interaction
The effect of other drugs on the active components of the Qlaira® drug
The interaction of PDAs with other drugs can lead to breakthrough uterine bleeding and / or lack of a contraceptive effect. The following types of interactions have been described in the literature on CPC in general or have been studied in the course of clinical trials of the drug Qlaira®:
Isozyme inducers (CYP3A4 isoenzyme). Interaction with drugs that induce microsomal enzymes (for example, cytochrome P450 systems) may occur, as a result of which the clearance of sex hormones can increase (such drugs include phenytoin, barbiturates, primidone, carbamazepine, rifampicin and, possibly, also oxcarbazepine, topiramate, felbamate, ritonavir, griseofulvin, and preparations containing St. John’s wort). It has been reported that HIV protease inhibitors (eg ritonavir), non-nucleoside reverse transcriptase inhibitors (eg nevirapine), and combinations thereof have also been reported.
Influence on intestinal-hepatic recirculation. While taking certain groups of antibiotics (for example, penicillin and tetracycline groups), enterohepatic circulation of estrogens may decrease, which can lead to a decrease in the concentration of estradiol.
Women who receive treatment with drugs that induce microsomal enzymes or antibiotics, in addition to Qlaira®, are advised to temporarily use a barrier method of contraception or choose another method of contraception. The barrier method of contraception should be used during the entire period of taking concomitant medications and for another 28 days after their withdrawal.
Isoenzyme inhibitors (CYP3A4 isoenzyme). Simultaneous administration of rifampicin together with tablets containing estradiol valerate and dienogest led to a significant decrease in Css and systemic exposure to dienogest and estradiol. Systemic exposure to dienogest and estradiol at Css, measured on the basis of AUC0-24, decreased by 83% and 44%, respectively.
Known inhibitors of CYP3A4, such as azole antifungals, cimetidine, verapamil, macrolides, diltiazem, antidepressants, and grapefruit juice, can increase the plasma concentration of dienogest. When taken simultaneously with a powerful inhibitor ketoconazole, the value of AUC0-24 in the equilibrium state for dienogest increased by 186%, and for estradiol – by 57%. With simultaneous use with a moderate inhibitor erythromycin, the value of AUC0-24 in dienogest and estradiol in the equilibrium state increased by 62 and 33%, respectively.
Effects of Qlaira® in relation to other drugs: CPC can affect the metabolism of a number of other drugs (for example, lamotrigine), which can lead to either an increase or a decrease in the concentration of these substances in blood plasma and tissues. However, based on data from in vitro studies, inhibition of CYP enzymes when using Qlaira® at a therapeutic dose is unlikely.
Note: to identify possible interactions, you should read the instructions for related drugs.
Incompatibility. Absent.
How to take, course of administration and dosage
How and when to take Qlaira
Take the tablets every day in the order indicated on the package, with or without food, at approximately the same time, with water if necessary. The pills are taken continuously. It should be taken one tablet a day consecutively for 28 days. Taking tablets from each new package begins after taking the last tablet from the previous calendar package. Menstrual bleeding usually starts while taking the last pills of the calendar pack and may not end until the next calendar pack starts. In some women, bleeding begins after taking the first tablets from a new calendar pack.
If used correctly, the Pearl index (an indicator reflecting the frequency of pregnancy in 100 women during the year of using the contraceptive) is less than 1. If pills are missed or misused, the Pearl index may increase.
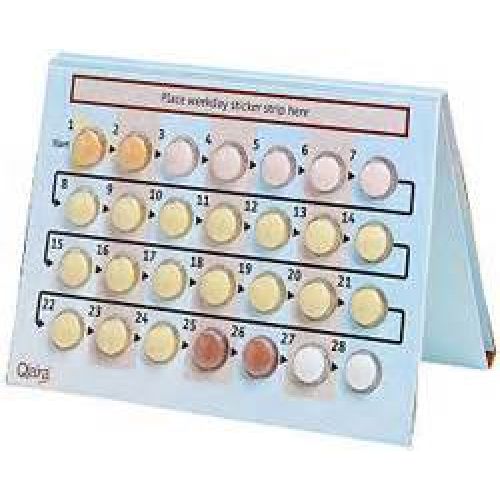
Preparing the book-packaging
To help you keep track of your pills, 7 stickers with the names of 7 days of the week are included in the package.
Select the sticker that begins on the day of the week that you start taking your pills. For example, if you start your appointment on a Wednesday, use a sticker that begins with the word “CP”.
Place this sticker at the top of Qlaira’s fold-out package where the “Place the weekday sticker here” is located, with the name of the first day above the number “1” pill.
\ Now, above each tablet there is the name of the corresponding day of the week, and you can see whether the tablet has already been taken on a particular day or not. Follow the direction of the arrow on the bookcase until you have consumed all 28 tablets.
Usually, so-called menstrual bleeding begins while you are taking the second dark red pill or white pills and may not be over before you start the next pack. For some women, bleeding still continues after taking the first pills in a new package.
Start the next package without interruption, i.e. the day after you have finished the current pack, even if the bleeding has not stopped. This means that the next pack should start on the same day of the week as the current one, and that menstrual bleeding should occur every month on the same days of the week.
If you use the Qlaira drug as indicated in the instructions, you are protected from unwanted pregnancy even during those 2 days when you are taking inactive pills.
How to start taking pills from the first pack?
- If you have not used hormonal contraceptives in the last month.
Start taking Qlaira on the first day of the cycle, i.e. on the first day of menstrual bleeding. - If you switch to taking Qlaira from other COCs, a combined contraceptive vaginal ring or patch.
Start taking Qlaira the next day after you drink the last active tablet (the last tablet with active ingredients) from the current pack of hormonal contraceptives. If the package of your previous contraceptives contains inactive pills, throw them away and continue taking from the first package of Qlaira without taking a break. If you have previously used a combined contraceptive vaginal ring or patch, start taking Qlaira on the day you remove the ring / patch, or follow your doctor’s advice. - When switching from a purely progestogenic method of contraception (mini-pills, an injection, an implant or an intrauterine system with the release of a progestogen (IUD)),
you can switch to taking Qlaira from a purely progestogenic method any day (from an implant or IUD – on the day of their removal; from the injection method – on the day on which the next injection is scheduled), but in all cases, during the first 9 days of taking Qlaira, you must use additional contraceptive measures (for example, condoms). - After a miscarriage
Consult your doctor. - After childbirth
If you have just undergone childbirth, the doctor may advise you to wait until your first normal periods have passed before using the Qlaira drug. Sometimes it is possible to start earlier. The doctor will advise you on how to proceed.
If you have had sexual intercourse after childbirth, make sure that there is no pregnancy or wait for the start of the next menstruation before starting to take Qlaira.
If you are breastfeeding and want to take Qlaira, please discuss this with your doctor first.
If you have any doubts about when to start taking Qlaira, seek advice from your doctor.
If too many Claira tablets have been drunk
The serious negative consequences of an overdose of Qlaira have not been reported.
If you take several active tablets at one time, you may develop nausea or vomiting. Young girls may have vaginal bleeding.
If you take too many Claira tablets, or find that a child has taken some of the tablets, seek advice from your doctor.
Steps to take in case of missing tablets
Tablets without active ingredients (placebo): If you miss one of the white tablets (2 white tablets at the end of the package), you do not need to take it later as it does not contain any active ingredients and you are still protected from unwanted pregnancy.
However, it is important that you discard the missed white tablet (s) and continue taking with the next tablet at the usual time. Otherwise, protection against unwanted pregnancy may be reduced (as a result of unintentionally prolonging the period during which active pills are not taken).
If you forget to take the last white tablet from the current package, it is important that you take the first tablet from the next package on time, nevertheless.
The following guidelines apply to skipping active tablets (1-26 tablets in a sachet box):
Active pills: Depending on the day of your menstrual cycle on which one active pill was missed, you may need to use additional contraceptive measures such as a barrier method such as condoms.
Take your tablets according to the following guidelines. See also the Missed Pills chart for details.
- If the delay in taking any pill is less than 12 hours, then the contraceptive effect of the drug remains. Take the pill as soon as you remember it, and take the rest of the pills at the usual time.
- If the delay in taking the pill is more than 12 hours, then the contraceptive reliability of the drug may decrease. Use additional contraceptive measures, such as a barrier method, such as condoms, depending on the day of your menstrual cycle. See also the Missed Pills chart for details.
- More than one tablet is missing from the package
Contact your healthcare professional for advice.
Do not take more than 2 active tablets in one day to make up for missed tablets.
If you forget to start a new pack, or missed one or more tablets from the 3rd to the 9th day of taking, there is a risk that you are already pregnant (if you had sexual intercourse within 7 days before the tablet was missed). In this case, contact your doctor. The more pills (especially with a combination of two active ingredients on days 3 to 24) are missed, and the closer they are to the inactive pill phase, the higher the likelihood of pregnancy. See also the Missed Pills chart for details.
If you forget to take any of the active tablets in the package and then do not bleed at the end of the package, you may be pregnant. Check with your healthcare professional before starting a new package.
What to do in case of vomiting or severe diarrhea
If, after taking any of the 26 active tablets of the Qlaira drug, you start vomiting or severe diarrhea, the absorption of the active substances may be incomplete. If vomiting occurs 3-4 hours after taking the pill, this is tantamount to missing the pill. Therefore, follow the recommendations in the section What to do if you miss a tablet. If you have severe diarrhea, see your doctor. Vomiting or diarrhea on the days of taking the last 2 inactive pills do not have any effect on the effectiveness of contraception.
You want to stop taking Qlaira
You can stop taking Qlaira at any time. If you are not planning a pregnancy, ask your doctor about other methods of contraception. If you want to get pregnant, stop taking Claira and wait for your natural menstrual bleeding before trying to get pregnant. This will help you calculate the expected date of birth of your baby.
If you have any further questions about the use of this drug, ask your doctor.
Overdose
Symptoms: no serious violations were reported in case of an overdose of Qlaira®.
Based on the total experience with the use of PDAs, there are symptoms that can occur with an overdose of active tablets: nausea, vomiting, spotting spotting or metrorrhagia.
Treatment: symptomatic.
Special instructions
If any of the diseases / conditions / risk factors listed below are currently available, then the potential risk and expected benefit of using the Qlaira® drug in each individual case should be carefully correlated and discussed with the woman before she decides to start taking drug. If any of these conditions or risk factors become worse, worse, or the first manifestation of any of these conditions or risk factors, a woman should consult with her doctor, who may decide whether to discontinue the drug.
Diseases of the cardiovascular system
The results of epidemiological studies indicate a relationship between the use of oral contraceptives and an increased incidence of venous and arterial thrombosis and thromboembolism (such as DVT, PE, MI and cerebrovascular disorders). The risk of developing venous thromboembolism (VTE) is highest in the first year of taking such drugs, mainly during the first 3 months. An increased risk is present after the initial use of the PDA or the resumption of the use of the same or different PDA (after a break between doses of the drug of 4 weeks or more). The overall risk of VTE in patients taking low-dose COCs (ethinyl estradiol content is less than 50 μg) is 2-3 times higher than in patients who do not take COCs, however, this risk remains lower compared to the risk of VTE during pregnancy and childbirth. VTE can be fatal (in 1–2% of cases).
VTE, manifested as DVT or PE, can occur with any PDA. Thrombosis of other blood vessels, for example, hepatic, mesenteric, renal, cerebral arteries and veins or vessels of the retina, occurs extremely rarely when using PDAs. There is no consensus on the connection between the occurrence of these events and the use of the PDA. Arterial thromboembolism can be fatal.
The risk of developing thrombosis (venous and / or arterial) and thromboembolism increases:
– with age;
– in smokers (with an increase in the number of cigarettes smoked or an increase in age, the risk increases, especially in women over 35 years old);
in the presence of:
– family history (for example, venous or arterial thromboembolism ever in close relatives or parents at a relatively young age). In the case of a hereditary or acquired predisposition, a woman should be examined by an appropriate specialist to resolve the issue of the possibility of taking the drug Qlaira®;
– obesity (body mass index more than 30 kg / m2);
– dyslipoproteinemia;
– arterial hypertension;
– migraine;
– heart valve diseases;
– atrial fibrillation;
– long-term immobilization; major surgery, any lower limb surgery or major trauma. In such situations, it is advisable to stop taking Qlaira® (for a planned operation – at least 4 weeks before it) and not resume taking it within 2 weeks after the end of immobilization.
The question of the possible role of varicose veins and superficial thrombophlebitis in the development of VTE remains controversial.
Consideration should be given to the increased risk of thromboembolism in the postpartum period. Peripheral circulatory disorders can also occur in diabetes mellitus, systemic lupus erythematosus, hemolytic uremic syndrome, chronic inflammatory bowel disease (Crohn’s disease or ulcerative colitis), and sickle cell anemia. An increase in the frequency and severity of migraines during the use of Qlaira® (which may precede cerebrovascular disorders) may be the basis for immediate discontinuation of this drug.
Biochemical factors that indicate a hereditary or acquired predisposition to arterial or venous thrombosis include the following: resistance to activated protein C, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies (anticardiolipid antibodies, lupus antibodies).
In assessing the risk / benefit ratio, it should be borne in mind that treating the condition in question may reduce the associated risk of thrombosis. It should also be borne in mind that the risk of thrombosis and thromboembolism during pregnancy is higher than with low-dose oral contraceptives (ethinyl estradiol content is less than 50 μg).
Tumors
The most significant risk factor associated with the development of cervical cancer is persistent human papillomavirus infection (PVI). There are reports of a slight increase in the risk of advanced cervical cancer with prolonged use of COCs. The connection with the reception of the PDA has not been proven. The possibility of interconnection of these data with screening for cervical diseases and features of sexual behavior (more rare use of barrier methods of contraception) is discussed.
A meta-analysis of 54 epidemiological studies revealed a slight increase in the relative risk (RR = 1.24) of developing breast cancer in women currently taking COCs. The increased risk gradually disappears over 10 years after you stop taking these drugs. Due to the fact that breast cancer is rare in women under 40 years of age, a slight increase in the number of diagnosed breast cancer in women who are currently taking COCs or have recently taken it is insignificant in relation to the overall risk of this disease. Its connection with the CCP has not been proven. The observed increase in risk may also be a consequence of an earlier diagnosis of breast cancer in women using COCs. Women who have ever used COCs have earlier stages of breast cancer than women who have never used them.
In rare cases, against the background of the use of CPC, the development of benign, and in extremely rare cases – malignant liver tumors was observed , which in some cases led to life-threatening intra-abdominal bleeding. In case of severe pain in the upper abdomen, an increase in the size of the liver, or signs of intra-abdominal bleeding in women taking COCs, it is necessary to exclude liver tumors in the differential diagnosis.
Other conditions
Women with hypertriglyceridemia (or a family history of this condition) may have an increased risk of developing pancreatitis while taking COCs.
Although a slight increase in blood pressure has been described in many women taking COCs, a clinically significant increase was rarely observed. However, if a persistent, clinically significant increase in blood pressure develops while taking Klaira®, the drug should be discontinued and treatment of arterial hypertension should be started. Taking the drug Qlaira®, if necessary, can be resumed if, through antihypertensive therapy, it is possible to achieve normal blood pressure.
The following conditions develop or worsen both during pregnancy and with the use of PDAs, but their relationship with the use of PDAs has not been proven: jaundice and / or cholestatic pruritus, cholelithiasis, porphyria, systemic lupus erythematosus, hemolytic uremic syndrome, Sydenham’s chorea, herpes of pregnant women hearing loss due to otosclerosis.
In women with hereditary forms of angioedema, exogenous estrogens can induce or worsen symptoms of angioedema.
Acute or chronic abnormalities in liver function may require discontinuation of Qlaira® until the liver function indicators return to normal. Recurrent cholestatic jaundice, which develops for the first time during pregnancy or the previous intake of sex hormones, requires discontinuation of Qlaira®.
Although COCs can influence insulin resistance and glucose tolerance, there is no need to change the therapeutic regimen in diabetic patients who use Qlaira®. Nevertheless, women suffering from diabetes mellitus need careful monitoring while taking Qlaira®.
Also described are cases of Crohn’s disease and ulcerative colitis against the background of the use of PDAs.
Chloasma can sometimes develop, especially in women with a history of pregnant chloasma.
Women who are prone to developing chloasma should avoid exposure to the sun or UV radiation while taking Qlaira®.
Impact on laboratory tests. Taking Klaira® can affect the results of some laboratory tests, including biochemical parameters of liver, thyroid, adrenal and kidney function, the concentration of transport proteins in plasma, such as CGH and lipid / lipoprotein fractions, parameters of carbohydrate metabolism, coagulation and fibrinolysis. These changes usually remain within laboratory limits.
Medical examinations. Before starting the use of the Qlaira® drug, it is necessary to carefully assess the contraindications to the administration of the drug based on the life history, family history of the woman, as well as general medical and gynecological examination. The frequency and nature of these examinations should be based on existing standards of medical practice with the necessary consideration of the individual characteristics of each patient. As a rule, blood pressure is measured, the condition of the mammary glands, abdominal cavity and pelvic organs is checked, including cytology of the cervix.
It is necessary to educate women that Qlaira® does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
Decreased efficiency. The effectiveness of the Klaira® drug can be reduced by skipping tablets with active ingredients (see recommendations for taking missed tablets in the section “Dosage and Administration”), gastrointestinal disorders while taking tablets with active ingredients (see recommendations for gastrointestinal disorders in the section “Dosage and Administration”) or against the background of concomitant drug treatment (see “Interaction”).
Insufficient control of the menstrual cycle. Against the background of the use of the drug Qlaira®, especially in the first months of admission, irregular menstrual bleeding may occur (spotting or breakthrough uterine bleeding). Therefore, the assessment of any irregular menstrual bleeding should only be done after an adaptation period of approximately 3 menstrual cycles.
If irregular menstrual bleeding recurs or occurs for the first time after previous regular cycles, the likelihood of non-hormonal causes should also be considered and a thorough examination should be carried out to exclude malignant neoplasms or pregnancy. Such activities may include diagnostic curettage.
Some women may not develop menstrual bleeding while taking inactive white pills. If you took the drug Qlaira® in accordance with the rules specified in the section “Dosage and Administration”, pregnancy is unlikely. However, if before the first absent menstrual bleeding the tablets were taken irregularly or there are no 2 menstrual bleeding in a row, you should not continue using the Qlaira® drug until pregnancy has been ruled out.
Impact on the ability to drive a car or perform work that requires an increased speed of physical and mental reactions. There was no negative effect of Qlaira® on the ability to drive and work with mechanisms, however, patients who have episodes of dizziness and impaired concentration during the adaptation period (the first 3 months of taking the drug) should be careful.
Storage conditions
Store at a temperature not exceeding 30 C.
Shelf life is 4 years.





















Reviews
There are no reviews yet.