Description
The instruction for medical use
of PRESTAHC medicine
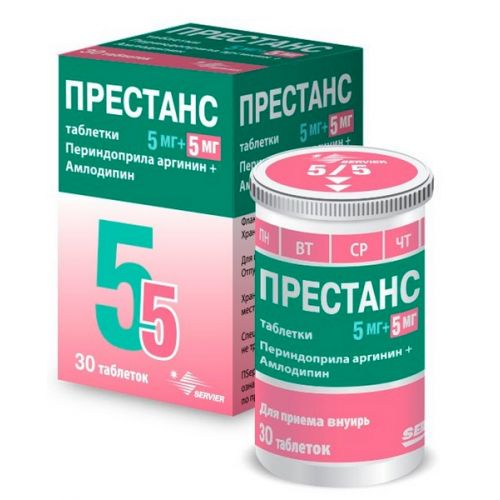
the Trade name
of Prestans
the International unlicensed name
Is not present
the Dosage form
of the Tablet, 5mg/5mg, 10mg/5mg, 5mg/10mg, 10mg/10mg
Structure
One tablet contains
active agents:
tablets 5mg/5mg: perindoprit arginine of 5 mg (3.395 mg
of a perindopril)
an amlodipina besilat 6.935 mg are equivalent (5 mg of an amlodipin are equivalent),
tablets 10mg/5mg: perindoprit arginine of 10 mg (
6.790 mg of a perindopril)
an amlodipina besilat 6.935 mg are equivalent (5 mg of an amlodipin are equivalent),
tablets 5mg/10mg: perindoprit arginine of 5 mg (3.395 mg
of a perindopril)
an amlodipina besilat 13.870 mg are equivalent (10 mg of an amlodipin are equivalent),
tablets 10mg/10mg: salt of 10 mg perindoprit arginine (
6.790 mg of a perindopril)
an amlodipina besilat 13.870 mg are equivalent (10 mg of an amlodipin are equivalent),
excipients: microcrystalline cellulose, lactoses monohydrate, magnesium stearate, silicon dioxide colloidal anhydrous.
Description
of Tablet 5mg/5mg: tablets of white color, the extended form, with marking on one party and the squeezed-out figures 5/5 – on other party
of Tablet 10mg/5mg: tablets of white color, triangular shape, with marking on one party and the squeezed-out figures 10/5 – on other party of Tablet 5mg/10mg: tablets of white color, square shape, with marking on one party and the squeezed-out figures 5/10 – on other party
of Tablet 10mg/10mg: tablets of white color, round shape, with marking on one party and the squeezed-out figures 10/10 – on other party
Pharmacotherapeutic group
the Drugs influencing a system renin-angiotensin. AKF inhibitors in a combination with other drugs. AKF inhibitors in a combination blockers of slow calcium channels. Perindopril and amlodipin.
The ATX C09BB04 code
the Pharmacological
Pharmacokinetics Pharmacokinetic Properties properties of a perindopril and amlodipin do not change in comparison with their separate use.
Perindopril
Vsasyvaniye and metabolism
After intake perindoprit quickly is absorbed. The maximum concentration (Cmax) is reached within 1 hour. The half-life period of a perindopril in plasma is is hydrolyzed with formation of an active metabolite of the perindoprilat 1 h 27% of the accepted dose of a perindopril. Other products of metabolism are inactive. Perindoprilat Cmax in blood plasma is reached in 3-4 h. Meal reduces transformation of a perindopril in perindoprilat and, therefore, its bioavailability. Communication between a dose of a perindopril and its exposure in plasma is linear.
Distribution
the Volume of distribution (Vd) of the free perindoprilat is close to 0.2 l/kg. Linking of the perindoprilat with proteins of blood plasma (generally about the angiotensin-the converting enzyme (ACE) makes 20% and depends on concentration of drug.
VyvedeniePerindoprilat is brought from an organism through kidneys. Elimination half-life (T1/2) of its free fraction makes about 17 h that allows to reach steady state in 4 days.
The pharmacokinetics in special clinical cases
At elderly patients and also at patients with renal and heart failure, removal of the perindoprilat slows down. At dialysis the clearance of the perindoprilat is 70 ml/min.
At patients with cirrhosis the hepatic clearance of an initial molecule of a perindopril slows down twice. However the number of the formed perindoprilat does not decrease therefore selection of a dose is not required.
Amlodipin
Vsasyvaniye and
Amlodipin’s metabolism is well soaked up from digestive tract, reaching Cmax in blood approximately in 6-12 hours after reception. The absolute bioavailability of drug is 64-80%. Meal does not affect bioavailability of an amlodipin. Amlodipin is substantially metabolized in a liver before formation of inactive metabolites.
Distribution
Linking with proteins of plasma makes about 97.5%.
Removal
of T1/2 makes 35-50 hours. 60% of the accepted dose are removed with urine, 10% in the form of not changed amlodipin.
The pharmacokinetics in special clinical cases
At elderly patients clearance of an amlodipin decreases that is followed by increase in AUC and T1/2.
At patients with a renal failure and abnormal liver functions the removal of an amlodipin slows down.
A pharmacodynamics
of Prestans – a combination of argininovy salt of a perindopril, inhibitor angiotensin – the converting enzyme, and an amlodipin, the antagonist of calcium ions of dihydropyridinic group.
Perindopril – AKF inhibitor. The converting enzyme or a kinase – the ekzopeptidaza turning angiotensin I into vasoconstrictive angiotensin II and causing disintegration of vasodilating compound of bradykinin to inactive heptapeptides. The inhibition of AKF leads to decrease in level of angiotensin II that leads to increase in activity of renin in plasma (by the principle of feedback) and to decrease in secretion of Aldosteronum.
As AKF blocks activity of bradykinin, the inhibition of AKF leads also to activation of a kallikrein-kinin system and system of prostaglandin.
Action of a perindopril is caused by activity of a metabolite of the perindoprilat. Perindopril is effective at any stage of arterial hypertension: soft, moderate and heavy. Decrease in the diastolic and systolic arterial blood pressure (ABP) occurs as in a prone position, and standing.
Perindopril reduces resistance of peripheral vessels that leads to decrease in the ABP. Thereof the peripheral blood stream increases, without influencing the heart rate (HR). The renal blood stream amplifies while the speed of glomerular filtration does not change.
The maximum of hypotensive effect is observed in 4-6 h after single dose and remains for 24 h. High degree of residual inhibition of activity of AKF in 24 h after administration of drug – 87-100% is noted.
At the patients sensitive to effect of drug, normalization of the ABP is not followed by reflex tachycardia. Drug withdrawal does not lead ment of hypertensive reaction.
Perindopril has vasodilating effect, restores elasticity of the main arteries, adjusts gistomorfologichesky changes in resistant arteries and causes regression of a hypertrophy of a left ventricle of heart.
Perindopril authentically reduces the cardiovascular mortality and frequency of development of complications.
Amlodipin – inhibitor of slow calcium channels, derivative dihydropyridine, blocking receipt of calcium ions through membranes in smooth muscles of a myocardium and vessels.
The mechanism of hypotensive action of an amlodipin is caused by the direct weakening effect on unstriated muscles of vessels.
Amlodipin expands peripheral arterioles and, thus, reduces the general peripheric resistance of vessels (GPRV), i.e. an afterload. As ChSS remains stable, reduction of an afterload leads to decrease in need of a myocardium for energy and oxygen.
Amlodipin expands the main coronary arteries and arterioles, including in ischemic zones of a myocardium that leads to increase in intake of oxygen in a myocardium at patients with vasospastic stenocardia (Printsmetal’s stenocardia or alternative stenocardia).
At patients with hypertensia the single daily dose of an amlodipin provides clinically significant decrease in the ABP for 24 hours as in a prone position, and standing. Thanks to the slow beginning of action amlodipin does not cause acute hypotension.
At patients with stenocardia the reception of a single daily dose of an amlodipin increases the general time of performance of physical activity, time of approach of an attack of stenocardia and development of a depression of a segment of ST detains (on 1 mm) during its performance, reduces the frequency of attacks of stenocardia and consumption of nitroglycerine.
Amlodipin has no adverse impact on a metabolism and level of lipids of blood plasma and can be appointed to patients with bronchial asthma, diabetes and gout.
Prestans authentically reduces the frequency of attacks of stenocardia, risk of developing a myocardial infarction and stroke and also cardiovascular mortality.
Indications
– arterial hypertension
– stable coronary heart disease
the Route of administration and doses
the Adult: Prestans accept inside on 1 tablet a day, it is desirable in the morning before food. The maximum single and daily dose – 1 tablet.
The fixed combination is not suitable for initial therapy. If necessary individual selection of a dose of Prestansa or replacement with a free combination is recommended.
Patients with disturbance of renal function and elderly patients
At the clearance of creatinine (CC) 60ml/mines dose adjustment is not required. For patients with clearance of creatinine & lt, 60ml/mines individual titration of a dose of separate components is recommended. Between change of plasma concentration of an amlodipin and extent of disturbance of renal function of correlation it is not observed.
Duration of therapy is established by the doctor individually.
Patients with a liver failure
of the Recommendation for patients with a slight and moderate abnormal liver function were not established therefore the dose should be chosen carefully and to begin reception with the lowest of the available doses. To define the optimum initial and supporting doses for patients with an abnormal liver function, it is necessary to carry out individual selection of doses with use of a free combination of an amlodipin and perindopril.
Side effects
Very often (≥1/10), it is frequent (≥1/100, & lt, 1/10), infrequently (≥1/1000, & lt, 1/100), is rare (≥1/10000, & lt, 1/1000), is very rare (≥1/10000), frequency is not established (on the basis of the available data it is not possible to carry out the development frequency assessment).
Often
– drowsiness, dizziness, vertigo, a headache, paresthesias, a dysgeusia
– face reddening (inflows), hypotension, tachycardia, pains in heart
– disorders of vision, sonitus
– the dry cough stopping at drug withdrawal, short wind
– nausea, vomiting, an abdominal pain, digestion disturbances, dyspepsia, diarrhea or a constipation
– a skin itching, rash
– muscular spasms, hypostasis of anklebones
– peripheral hypostases, feeling of fatigue, an asthenia
Infrequently
– insomnia, a hypesthesia, sleep disorders, differences of mood, a tremor, a temporary loss of consciousness (syncope), a depression, an indisposition
– congestion of a nose, rhinitis, a bronchospasm
– a dysuria, a night polyuria, a renal failure
– an arthralgia, myalgia, a dorsodynia and a thorax
– allergic reactions, a purpura, urticaria, a Quincke’s disease
– impotence, a gynecomastia, an alopecia, a skin depigmentation, the increased sweating
– dryness in a mouth, changes in an intestines vermicular movement
– loss and weight reduction
Is rare
– increase in level of bilirubin and liver enzymes in blood serum
– confusion of consciousness
Is very rare
– peripheral neuropathy
– a vasculitis, arrhythmia (including bradycardia, ventricular tachycardia and fibrillation of auricles), the myocardial infarction and a stroke (which are perhaps connected with the profound hypotension at patients of risk group), stenocardia
– eosinophilic pneumonia
– a hypertrophic ulitis, gastritis, hepatitis, cholestatic jaundice, pancreatitis, increase in indicators of liver enzymes (mainly connected with a cholestasia)
– an acute renal failure
– a leukopenia, a neutropenia, thrombocytopenia, an agranulocytosis or a pancytopenia, hemolytic anemia at patients with congenital insufficiency of glyukozo-6-fosfatdegidrogenazy (G-6FDH), decrease in hemoglobin and a hematocrit
– a hyperglycemia
– a hypertension, peripheral neuropathy
– a Quincke’s edema
– a multiformny erythema, Stephens-Johnson’s syndrome (malignant exudative erythema), exfoliative dermatitis, photosensitivity
Frequency is not determined
– increase in level of urea in blood and creatinine in blood serum, a hyperpotassemia
– a hypoglycemia
there Are single messages of an extrapyramidal syndrome.
Contraindications
– hypersensitivity to the perindopril, an amlodipin, other AKF inhibitors derivative of dihydropyridine and other components of drug
– a Quincke’s disease against the background of the previous therapy by AKF inhibitors in the anamnesis
– a hereditary or idiopathic Quincke’s disease
– the profound hypotension
– shock, including cardiogenic
– obstruction of an output path of a left ventricle (for example, a considerable stenosis of an aorta)
– heart failure after an acute myocardial infarction
– pregnancy and the period of a lactation
Medicinal interactions
AKF Inhibitors reduce the potassium loss caused by diuretics. Kaliysberegayushchy diuretics (for example, Spironolactonum, Triamterenum or amiloride), can lead potassium additives or kaliysoderzhashchy substitutes of salt to significant increase in content of potassium in serum. If the accompanying use in view of the expressed hypopotassemia is shown, drugs have to be used with extra care and with frequent monitoring of content of potassium in serum.
At the combined intake of lithium and AKF inhibitors, cases of increase in concentration of lithium in serum and development of toxic effect of lithium are noted. The combined reception of a perindopril and drugs of lithium is not recommended, but if necessary it is necessary to control carefully lithium content in blood serum.
Joint reception with estramustinum can lead to increase in risk of developing a Quincke’s disease.
Non-steroidal anti-inflammatory drugs (NPVS), including acetylsalicylic acid in a dose 3g/put, and AKF inhibitors render additive effect on increase in level of potassium in blood plasma, leading to deterioration in function of kidneys. The specified action has reversible character, but the acute renal failure, especially at patients with a renal failure, for example, at elderly patients in rare instances can develop or at an obezvozhennost. At such patients before an initiation of treatment it is necessary to estimate functional activity of kidneys and, if necessary, to provide sufficient hydration of an organism.
Joint intake of AKF inhibitors and antidiabetic drugs (insulin, oral hypoglycemic drugs) can lead to strengthening of hypoglycemic effect. The hypoglycemia comes seldom (probably improvement of tolerance of glucose with the subsequent decrease in need for insulin).
At the patients taking the diuretic drugs, especially at patients with decrease in the volume of the circulating blood (VCB) and/or electrolytic disturbances after the beginning of therapy the profound lowering of arterial pressure can be observed by AKF inhibitor. Diuretic cancellation, completion of OCK or correction of electrolytic balance before an initiation of treatment and also purpose of low initial doses of a perindopril and their gradual increase reduce risk of developing hypotension.
Sympathomimetic drugs can reduce hypotensive effect of AKF inhibitors.
Simultaneous therapy by AKF inhibitors and drugs of gold (aurotiomalat sodium) can lead ment of nitritoidny reactions (face reddening, nausea, vomiting and hypotension).
It is necessary to avoid the combined purpose of an amlodipin and a dantrolen in connection with risk of development of fibrillation of ventricles with a possible lethal outcome.
The inductors CYP3A4 (rifampicin, drugs of a St. John’s wort of made a hole (Hypericum perforatum), carbamazepine, phenobarbital, Phenytoinum, fosfenitoin, Primidonum) lead to decrease in plasma concentration of an amlodipin. It is necessary to be careful at combination of an amlodipin with the inductors CYP3A4, if necessary the dose of an amlodipin can be adapted.
The accompanying reception of an amlodipin with CYP3A4 inhibitors (itrakonazol, ketokonazol) can lead to increase in plasma concentration of an amlodipin and development of side effects. It is necessary to be careful at combination of an amlodipin with CYP3A4 inhibitors, if necessary the dose of an amlodipin can be adapted.
Due to the risk of developing hypotension and deterioration in function of a myocardium at patients with latent or uncontrollable heart failure the care when assigning β-blockers is necessary (bisoprolol, karvedilol, metoprolol) and vazodilatator with Prestansom. Besides, β-blockers suppress function of a sympathoadrenal system in the profound hemodynamic repercussion.
Baclofenum enhances expressiveness of hypotensive effect (it is necessary to control the ABP level and to adjust a dose of Prestansa).
The concomitant use of Prestansa with hypotensive drugs of other groups, vazodilatator, nitroglycerine, other nitrates can lead to strengthening of hypotensive effect.
Corticosteroids and tetrakozaktid reduce hypotensive action of Prestansa (the delay of water and salt caused by action of corticosteroids).
Amifostin strengthens hypotensive action of an amlodipin.
The combined reception with tricyclic antidepressants, antipsychotic drugs, the anesthetizing drugs and ά-blockers (Prazozinum, alfuzozin, docsazozin, tamsulozin, terazozin) can lead to strengthening of hypotensive effect and increase in risk of developing orthostatic hypotension.
Special instructions
At elderly patients and patients with a renal failure the removal of drug is slowed down in this connection it is necessary to control the level of potassium and creatinine often.
It is necessary to appoint with care drug to patients with abnormal liver functions. In rare instances intake of AKF inhibitors was followed by development of cholestatic jaundice with progressing in the fulminantny necrosis of a liver which sometimes is coming to an end with a lethal outcome. The mechanism of this syndrome is not clear. Patients at whom jaundice develops or considerably the level of hepatic enzymes increases, have to stop intake of AKF inhibitor.
Rare messages about a Quincke’s disease of the face, lips, mucous membranes, language, a glottis and/or throat, extremities at the patients accepting AKF inhibitors arrived, including perindoprit. In a paraglossa, a glottis or a throat at which obstruction of airways is probable, it is necessary to give immediately first aid which can include prescribing of adrenaline and/or maintenance of free airways. The patient has to be under medical observation before total and final disappearance of symptoms. There are separate messages about the Quincke’s disease of intestines which is shown an abdominal pain, nausea, vomiting or without them. Symptoms stopped after cancellation of intake of AKF inhibitor. Intestinal angiootek it is necessary to differentiate with pains of other etiology.
During a procedure of an aferez of lipoproteins of the low density (LDL) using dextran sulfate the risk of emergence of anaphylactoid reactions at patients increases. It is necessary to avoid prescribing of AKF inhibitors to such patients. Reception of Prestansa is stopped one day before the beginning of an aferez of LDL.
It is necessary to show care when assigning Prestansa to patients with allergic diseases and taking a course of the desensibilizing therapy and also to avoid its use when carrying out an immunotherapy allergens from poison of insects. For the purpose of reduction of risk of development of anaphylactoid reactions the therapy of Prestansom is stopped at least one day before the beginning of a course of the desensibilizing therapy.
Neutropenia/agranulocytosis/thrombocytopenia/anemia
At the patients accepting APF inhibitors the neutropenia/agranulocytosis, thrombocytopenia and anemia were noted. At patients with normal function of a liver and lack of other complicating factors, the neutropenia comes seldom. At reception of a perindopril, patients should observe extreme care with vascular diseases and immunodepressants undergoing therapy, treatment by Allopyrinolum or procaineamide, or that who has all these complicating factors, in particular, at already available abnormal liver functions. At some of such patients serious infections developed. In some cases intensive care by antibiotics was unsuccessful. When assigning a perindopril such patients are recommended to carry out periodic monitoring of calculation of leukocytes and to instruct patients about need to report about any symptoms of an infection (e.g., a sore throat, heat).
Use for patients with an abnormal liver function
At patients with an abnormal liver function the elimination half-life of an amlodipin and AUC value increase. The recommended doses for them are not established therefore these patients need to begin purpose of an amlodipin with low doses and to be careful, both at primary treatment, and at increase in a dose. Patients with a heavy abnormal liver function should carry out consistently selection of a dose.
AKF inhibitors can cause falling of the ABP, renin – dependent hypertensia is more often at patients with a reduced volume of the circulating blood (accepting diuretics, being on a diet with the limited content of salt, the patients on dialysis having vomiting or diarrhea) or at patients from heavy. At such patients it is necessary to control carefully the ABP level, potassium in serum and functional activity of kidneys. In case of development of hypotension it is necessary to put the patient on a back and, if necessary, intravenously to enter 0.9% sodium chloride solution.
As well as other AKF inhibitors, perindoprit it is necessary to appoint with extra care to patients with a stenosis of the mitral valve and obstruction of an output path of a left ventricle (stenosis of an aorta or a hypertrophic cardiomyopathy).
In the presence of a renal failure (clearance of creatinine & lt, 60ml/mines) individual selection of doses of separate components is recommended.
At some patients with a bilateral renal artery stenosis or a stenosis of an artery of the only kidney accepting AKF inhibitors cases of passing increase in level of urea in blood and creatinine in serum are noted. In renovascular hypertensia there is also a risk of developing heavy hypotension and renal failure. In need of prescribing of drug of this category of patients it is necessary to control the level of potassium and creatinine regularly.
At patients of negroid race the hypotensive action of a perindopril can be less significant, than at patients of other race (perhaps, it is connected with the fact that hypertensia at patients of negroid race very often proceeds against the background of the low level of renin).
During treatment by AKF inhibitors the patient can have a dry unproductive cough which stops after drug withdrawal.
At the planned surgical intervention with anesthesia it is necessary to stop treatment of Prestansom in 1 day prior to operation.
Cases of increase in level of potassium in blood serum at patients with a renal failure, elderly patients (70 years), the patients with diabetes, a hypovolemia, a decompensation of warm activity, a metabolic acidosis accepting kaliysberegayushchy diuretics (Spironolactonum, eplerenon, Triamterenum or amiloride are more senior), potassium additives or kaliysoderzhashchy substitutes of salt and also other drugs increasing potassium level (heparin), especially in the presence of abnormal liver functions are noted. The accompanying use of a perindopril with the specified drugs has to be carried out with care and at regular monitoring of level of potassium in blood serum.
It is regularly necessary to carry out monitoring of level of a glycemia at the patients taking oral glucose-lowering drugs and insulin.
Amlodipin with care appoint in heart failure, in connection with possible risk of developing a fluid lungs.
Drug contains lactose therefore it should not be applied in a congenital galactosemia, a glyukozo-galaktozny malyabsorbtsionny syndrome and also at deficiency of Lappa lactase.
Reception of an amlodipin with grapefruit or grapefruit juice as at some patients it can lead to increase in its bioavailability and, therefore, to strengthening of hypotensive effect is not recommended.
Use in pediatrics
the Efficiency and safety of use of Prestansa at children’s and teenage age (up to 18 years) is studied insufficiently in this connection prescribing of drug of this category of patients is not recommended.
The feature of influence on ability to control of motor transport and potentially dangerous mechanisms
Should be careful during the driving of motor transport and work with potentially dangerous mechanisms in connection with possible development of the individual reactions connected with decrease in the ABP, especially at the initial stage of treatment or at combination therapy with hypotensive drugs.
Overdose
Symptoms: hypotension, circulator shock, disturbances of electrolytic balance, a renal failure, a hyperventilation, tachycardia, the strengthened heartbeat, bradycardia, dizziness, uneasiness and cough. The available data show that the dressed-up overdose can lead to the strengthened expansion of peripheral vessels and, perhaps, to reflex tachycardia. There are messages about expressed and, perhaps, long system hypotension up to / and including shock with a lethal outcome.
Treatment: Led gastric lavage, intake of activated carbon within 2 hours after reception of an amlodipin of 10 mg to decrease in level of absorption of an amlodipin.
In the clinically apparent hypotension caused by overdose of an amlodipin the active support of a cardiovascular system, including frequent control of warm and respiratory function, observation of OCK and volume of the emitted urine is necessary. It is also necessary to raise the lower extremities.
Vasoconstrictive drugs can help to restore a vascular tone and arterial blood pressure, on condition of lack of contraindications. Intravenous administration of a gluconate of calcium can be useful to elimination of consequences of blockade of calcium channels.
As amlodipin well contacts proteins of plasma, the effect of dialysis is represented improbable.
At overdose it is recommended to carry out intravenous infusion of isotonic normal saline solution. In case of development of hypotension of the patient it is necessary to put in horizontal position. At an opportunity, it is necessary to consider treatment option angiotensin II in the form of infusion and/or intravenous administration of catecholamines. Perindopril is brought from system blood circulation by a hemodialysis. In case of the bradycardia resistant to therapy, carrying out electrocardiostimulation is shown. It is necessary to control constantly the vital indicators, level of electrolytes and creatinine in serum.
A form of release and packing
On 30 tablets in polypropylene tubas, with the dosing opening for gradual delivery of tablets. The tuba is closed by a stopper from polyethylene of the low density containing 2 g of siccative gel. On 1 tuba together with the instruction for medical use in the state and Russian languages put in a cardboard pack.
To Store storage conditions at a temperature not above 30 °C.
To store out of children’s reach!
A period of storage
2 years
not to use drug after the expiration date specified on packing.
Prescription status
According to the prescription
Servier (Ireland) Industries Ltd Producer (Servye (Ireland) Industries Ltd), Ireland
the Owner of the registration certificate
of Les Laboratoires Servier (Le Laboratoir of Servye), France
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
Representation Le Laboratoir of S.A.S. Servye. in PK
050020, Almaty, Dostyk Ave of 310 g, Business center, the 3rd floor
Ph.: (727) 386 76 62/63/64/70/71
Fax: (727) 386 76 67
E-mail:
kazadinfo@kz.netgrs.com














Reviews
There are no reviews yet.