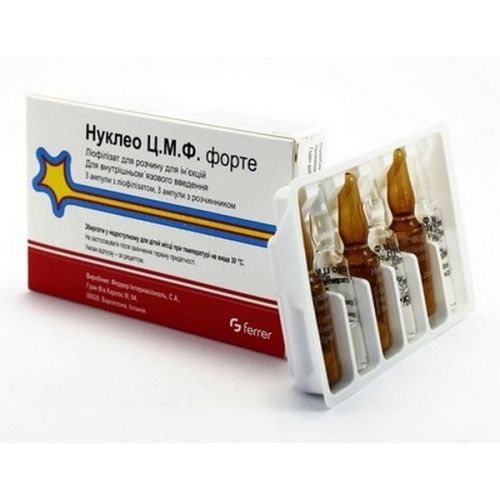
Nucleo TS.M.F. forte 2ml 3’s lyophilized powder for quality in with the solvent solution
$18.90
Description
Instruction for medical use
of Nukleo medicine of Ts.M.F. Forte
Trade name
of Ts.M.F. Nukleo. FORTE
the International unlicensed name
Is not present
the Dosage form
the Powder lyophilized for preparation of solution for intramuscular introduction complete with
the Structure One Ampoule solvent of drug contains
active agents: – cytidine 5 dinatrium monophosphate 10.00
uridine 5′ – trisodium triphosphate
uridine 5 ‘-dinatrium
uridine 5 diphosphate’ – dinatrium monophosphate (only) 6.00
(it is equivalent to uridine 2.66 content)
excipients: Mannitolum
Composition of solvent: chloride sodium, water for an injection.
The description
the Powder lyophilized white or almost white color is hygroscopic.
Solvent – flavourless transparent colourless liquid.
Pharmacotherapeutic group
Drugs for treatment of diseases of nervous system other
the ATX N07XX Code
the Pharmacological
Pharmacokinetics Components properties of this drug have organic origin which are already present at biological liquids, causes difficulty of carrying out pharmacokinetic researches of drug, and it formed the basis of carrying out a research with use of a product, marked radioactive isotopes. The purposes of this research consisted in assessment of absorption and pharmacokinetics of UMF, CMF and also UTF.
After use 14S-UMF a dose of 4.97 mg/kg, the maximum concentration in plasma (Cmax) making 1.749 mg/kg were reached in 10 minutes. Then radioactivity was quickly removed with elimination half-life 0.5 and 0.6 hours for alpha distribution and beta elimination, respectively.
After use 14S-TsMF a dose of 4.83 mg/kg, the Cmax value, the making 1.567 mg/kg, were reached in 20 minutes. Then radioactivity was quickly removed with the corresponding elimination half-life 1.0 and 3.8 hours.
After use 14S-UTF a dose of 4.86 mg/kg, the Cmax value, the making 1.291 mg/kg, were reached in 20 minutes. Then radioactivity was quickly removed with the corresponding elimination half-life of 1.2 and 5.0 hours.
The same way of metabolism was found in UMF and UTF, i.e. fast transformation through uracil to, at least, one more polar radioactive fraction.
The CMF connection quickly turned in tsitozin, uracil and, at least, one more polar radioactive fraction. Therefore tsitozin, generally turned into uracil. These results indicate that in blood less fast metabolism of uracil in the presence of a tsitozin who was formed of the initial accepted connection – CMF was observed.
Nukleo’s pharmacodynamics of CMF Forte contains a number of nucleotides: cytidine monophosphate (CMF), uridine triphosphate (UTF) which widely use for treatment of diseases PNS (peripheral nervous system).
From the biochemical point of view, action of these two components can be described as follows: CMF takes part in synthesis of a complex of lipids which form a neuronalny membrane, mainly sphingomyelin — the main component of a myelin cover. CMF is also a predecessor of nucleic acids (DNA and RNA) which, in turn, are basic elements of cellular metabolism (for example, in proteinaceous synthesis). UTF works as a coenzyme in synthesis of glycolipids, neuronalny structures and a myelin cover, supplementing action of CMF. Besides, he acts as a power source in the course of reduction of muscles. Generally, CMF and UTF take part in synthesis of phospholipids and glycolipids which, generally make a myelin cover and other nervous structures. It results in the intensive metabolic activity promoting, in turn, process of regeneration of a myelin cover, regulating demyelination at peripheral nervous damages. Thus, association of action of CMF and UTF promotes regeneration of a myelin cover, the correct carrying out nervous excitement and restoration of a muscular trophicity. Ts.M.F. Nukleo. FORTE provides an organism with the phosphatic groups necessary for association of monosaccharides with Keraminums for forming of nervous covers, and the fosfatidny acids making sphingomyelin and glycerophospholipids – the main components of a myelin cover. Thus, the steady trophic effect, fuller maturing and regeneration of axonal nerve fibrils is provided. Therefore inflammation decreases and the sensitivity of the damaged site of an axon is normalized that promotes restoration of axonal transport.
Indications
– an ischialgia
– sciaticas
– diabetic polyneuropathy
– poluneuritis potatorum
– shingles
– neuralgia front, trigeminal, intercostal nerves
– a lumbago.
The Powder lyophilized to dissolve a route of administration in 2 ml of solvent and to enter intramusculary right after cultivation.
Adult: on 2 ml (1 ampoule) once a day.
A course of treatment up to 21 days.
To children since 2 years: 2 ml (1 ampoule) of 1 times in 2 days.
Course of treatment of 6 – 12 days.
Side effects
were Not described, but in case of any adverse reactions connected with drug use consult with your attending physician.
Contraindications
– hypersensitivity to drug components
– children’s age up to 2 years
Medicinal interactions
are not known
the Special
instructions Pregnancy and the period of a lactation.
The expediency of reception is established depending on prevalence of advantages of reception over potential risk
of Feature of influence on ability to run motor transport or other potentially dangerous mechanisms
the Overdose
Due to the small toxicity of drug does not influence the probability of overdose is small.
The form of release and packing
place the Powder lyophilized in ampoules of orange neutral glass with a ring for opening.
On 2 ml of solvent in ampoules colourless neutral steklas a ring for opening.
On 3 ampoules with drug and 3 ampoules with solvent put in blister strip packaging from a film half-vinylchloride.
On 1 blister strip packaging together with the instruction for medical use in the state and Russian languages put in a pack from cardboard.
To Store storage conditions at a temperature not over 300C.
To store out of children’s reach!
2 years
not to use a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer Ferrer Internasional, S.A.,
Gran Via Carlos III, 94, 08028, Barcelona, Spain.
Owner of the registration certificate
Ferrer Internasional, S.A.,
Gran Via Carlos III, 94, 08028, Barcelona, Spain.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
Representation in RK:
Medial Dee and Pi LTD across Central Asia in the Republic of Kazakhstan
Address: 050050 Almaty, Tabachnozavodskaya St., 20, ph. +77272954882/83, the fax – 2954881
e-mail: medial.office@gmail.com

















Reviews
There are no reviews yet.