Description
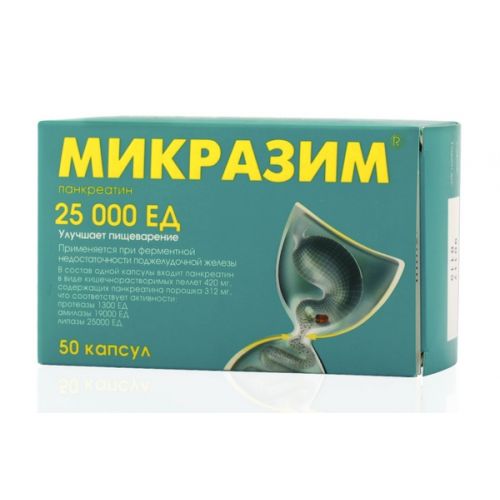
The instruction for medical use of MIKRAZIM® medicine Trade name МИКРАЗИМ® the International unlicensed name Is not present the Dosage form of the Capsule 10000 PIECES and 25000 PIECES Structure of 10000 PIECES 25000 PIECES One capsule contains active agent – Pancreatinum in the form of the kishechnorastvorimy pellets containing powder Pancreatinum that corresponds to activity: proteases of amylase of a lipase of 168 mg * 125 mg 520 PIECES 7500 PIECES 10000 PIECES 420 mg * 312 mg 1300 PIECES 19000 PIECES 25000 PIECES * – in terms of nominal lipolytic activity. excipients cover of pellets: methacrylic acid and ethyl acrylate copolymer [1:1] (in the form of 30% of the dispersion which is in addition containing polysorbate-80, sodium lauryl sulfate) – 25.3 mg / 63.2 mg, triethyl citrate – 5.1 mg / 12.6 mg, simetikon an emulsion of 30% (dry weight, including: dimetikon, the silicon colloidal besieged, silicon colloidal weighed, methyl cellulose, sorbic acid, water) – 0.1 mg / 0.3 mg, talc – 12.6 mg / 31.6 mg, a capsule cover: for a dosage of 10000 PIECES: ferrous oxide yellow E172 – 0.2240%, ferrous oxide black E172 – 0.3503%, ferrous oxide red E172 – 0.8077%, the titan E171 dioxide – 0.6699%, gelatin – up to 100%, for a dosage of 25000 PIECES: red charming E129 – 0.1400%, ferrous oxide yellow E172 – 0.3000%, the titan E171 dioxide – 0.5000%, gelatin – up to 100%. The description Solid gelatin capsules No. 2 with the transparent body and a cover of brown color (for a dosage of 10000 PIECES) or size No. 0, with the transparent body and a cover of dark orange color (for a dosage of 25000 PIECES). Contents of capsules – pellets of cylindrical or spherical or irregular shape from light brown till brown color, with a characteristic smell. Pharmacotherapeutic group Drugs, digestant (including fermental drugs). Digestive fermental drugs. Pancreatinum. ATH A09AA02 code the Pharmacological Pharmacokinetics Pancreatinum properties – the drug emitted from a pancreas of animals. МИКРАЗИМ® contains Pancreatinum of pork origin. Drug contains mainly high-molecular fermental proteins, insignificant amount of mineral substances. In researches on animals the lack of absorption of the whole (not split) enzymes was shown and thereof, classical pharmacokinetic researches were not executed. As the therapeutic activity of the drugs containing pancreas enzymes is implemented in a GIT gleam, absorption is not required for manifestation of their effects. Moreover, on the chemical structure, enzymes are proteins and, in this regard, when passing through a GIT are exposed to proteolytic splitting until they are not absorbed in the form of peptides and amino acids. The pharmacodynamics Digestive fermental means, fills shortage of enzymes of a pancreas, has lipolytic, proteolytic, amylolytic effect. After administration of drug the gelatin capsule under the influence of gastric juice is dissolved in a stomach and Pancreatinum pellets resistant to gastric acid, easily mix up with contents of a stomach and together with the digested food get into a small intestine. Here pellets lose an acid resisting cover, break up and release the active enzymes promoting active digestion of components of food in an intestines gleam. The lipase promotes splitting of fats to glycerin, hydrolyzing radio bonds in provisions 1 and 3 of triglycerides of fatty acids. Alpha amylase hydrolyzes alpha 1.4 – glikozidnye glucose polymers. Splits mainly extracellular polysaccharides (starch, a glycogen and some other carbohydrates) and practically does not participate in cellulose hydrolysis. Starch and pectins decomposes to simple sugars – sucrose and maltoses. Proteolytic enzymes – trypsin, chymotrypsin and elastase – split proteins to amino acids. Besides, trypsin, destroying a cholecystokinin-rileasing-factor, by the principle of feedback inhibits stimulated food secretion of a pancreas that reduces load of this body and at the expense of it the anesthetizing action in acute pancreatitis is provided. Trypsin, interacting with RAP-2-retseptorami of enterocytes, is the important factor regulating motility of a small intestine. Drug improves a functional state gastrointestinal a path, normalizes digestion processes. Unlike Pancreatinum tablets, the microgranulated form of Pancreatinum provides fast passing of drug to a duodenum from a stomach, the maximum enzymatic activity of drug in a small intestine is registered in 30-45 min. after oral administration. In lower small intestines the activity of enzymes of Pancreatinum sharply decreases, in process of advance in digestive tract there is their inactivation and partial degradation, residues of drug are brought out of intestines together with products of digestion of food. Indications With the replaceable purpose at exocrine insufficiency of a pancreas – a mucoviscidosis – chronic pancreatitis – a state after pancreatectomy – a pancreatic cancer – a state after a full or partial resection of a stomach (a gastroenterostom) – obstruction of pancreat ducts or the general bile duct (including, owing to a new growth) – Shvakhmana-Diamond’s syndrome – acute pancreatitis during restoration of an enteroalimentation select the Route of administration and doses of the Dose of drug for Billroth-II individually. The drug dose (in terms of a lipase) depends on age and degree of fermental insufficiency. Consider also relative content of the enzymes hydrolyzing proteins and carbohydrates depending on structure of a diet and associated diseases. Adults take the drug during meal. Capsules are swallowed entirely, without breaking and without chewing, washing down with enough water. Not to use alkaline mineral water for a zapivaniye. If the single dose makes more than one capsule, it is necessary to accept about a half or one third of the recommended single dose before meal, the rest – at meal time. For administration of drug by the adult with the complicated swallowing and to children the capsule should be opened and added a pellet to food which does not demand chewing (porridge, apple puree, yogurt, etc.). The prepared mix should be accepted at once. Crushing or chewing of pellets leads to disturbance of their acid resisting cover, the released pancreatic enzymes quickly lose activity and, besides, can cause irritation of a mucous membrane of a mouth and gullet. Mukovistsidozirovanny the Initial calculated dose for children is younger than 4 years makes 1000 PIECES of a lipase on kilogram of body weight at each feeding, for children 4 years – 500 PIECES of a lipase on kilogram are more senior at each meal. The dose should be selected individually, depending on disease severity, expressiveness of a steatorrhea and the nutritive status. The maintenance dose for most of patients should not exceed 10,000 PIECES of a lipase on kilogram of body weight a day. The day dose can be divided into several receptions with intervals of 1-2 hours. As it is difficult to divide contents of the capsule into several receptions, the PIECE МИКРАЗИМ® 10000 drug treatment is recommended to begin at children with body weight not less than 10 kg, the PIECE МИКРАЗИМ® 25000 drug treatment is recommended to begin at children with body weight not less than 25 kg. Other types of exocrine insufficiency of a pancreas. At replacement therapy at patients with chronic pancreatitis of a dose of enzymes select individually depending on degree of vneshnesekretorny insufficiency and also individual eating habits of the patient. At considerable contents (more than 15 grams a day) fat in Calais and also in the presence of ponos and decrease in body weight when the diet does not give essential effect – appoint 25000 units of a lipase (contents of one PIECE МИКРАЗИМ® 25000 capsule) at each meal. If necessary and at good tolerance of drug the single dose is raised to 30000 – 35000 (three capsules of drug МИКРАЗИМ® 10000 PIECE or on one PIECE МИКРАЗИМ® 10000 capsule and МИКРАЗИМ® 25000 PIECES, respectively). Further increase in a dose, in most cases, does not improve treatment outcome and demands revision of the diagnosis, decrease in content of fat in a diet. Side effects Very often − an abdominal pain * it is frequent – nausea, vomiting, a meteorism, diarrhea, a constipation Infrequently – rash Frequency is unknown – a fibroziruyushchy colonopathy, reactions of hypersensitivity (anaphylactic reactions), skin allergic reactions: urticaria, an itching * Gastrointestinal disorders are connected with a basic disease. Frequency of an abdominal pain and diarrhea is similar or below, than in the group accepting placebo. The Fibroziruyushchy colonopathy is described at the patients with a mucoviscidosis accepting high doses of the drugs containing Pancreatinum In clinical trials with participation of patients of children’s age additional side reactions are not revealed. Contraindications – individual intolerance of Pancreatinum or separate components of drug – chronic pancreatitis in an aggravation stage – acute pancreatitis Medicinal interactions At simultaneous use of Pancreatinum with preparations of iron, folic acid the decrease in absorption of the last is possible that demands increase in a dose. Miglitol (diastabol), acarbose and other hypoglycemic oral drugs – inhibitors of glucosidases. The amylolytic activity of enzymes of Pancreatinum can weaken or completely remove therapeutic effect of these drugs at patients with diabetes (2 types). Joint reception МИКРАЗИМА® and oral hypoglycemic means is not recommended as increases risk of a hyperglycemia at such patients. The antiacid means containing calcium a carbonate and/or magnesium hydroxide. Simultaneous use with МИКРАЗИМОМ® can cause decrease in efficiency of the last owing to increase rn environments of a stomach and premature release and destruction of enzymes of drug. Special instructions Children can take the drug only in the form of contents of the opened capsules. Replacement therapy of exocrine function of a pancreas should not replace or postpone treatment of primary cause of illness. The children and adults receiving long-term treatment by the drug MIKRAZIM® high doses have to be observed regularly at the expert. Are basic reasons of inefficiency of fermental therapy: an inactivation of enzymes in a duodenum as a result of acidulation of its contents, associated diseases of a small intestine (helminthic invasions, disbioz guts, etc.), duodenostaz, non-performance by patients of the recommended treatment mode, use of the enzymes which lost the activity (at disturbance of the recommended storage conditions or use of drug upon termination of the term of its validity). Pregnancy and the period of a lactation Data on potential risks of use of Pancreatinum at pregnant women and in the period of a lactation are absent therefore pregnant and nursing mothers should appoint drug only if the useful effect exceeds possible risks. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms Drug does not affect ability to driving of the car and to control of cars and mechanisms. Overdose Symptoms: increase in content of uric acid in urine (hyperuricuria) and blood (hyperuricemia). Treatment: drug withdrawal, symptomatic therapy. Form of release and packing of the Capsule 10000 PIECES and 25000 PIECES. On 10 capsules in blister strip packaging from a film of the polyvinylchloride and printing aluminum foil varnished. On the 2 or 5 blister strip packagings together with the instruction for medical use in Russian and state languages place in a pack from cardboard. To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C. Period of storage 2 years. To apply N after expiry date. Prescription status Without prescription. Producer/packer: JSC AVVA RUS, Russia 610044, Kirov, Luganskaya St., 53a. Ph.: +7 (8332) 25-12-29, + 7 (495) 956-75-54 avva.com.ru the Name and the country of the owner of the registration certificate of JSC AVVA RUS, Russia the Address of the organization in the territory of the Republic of Kazakhstan, the accepting claim (offer) on quality of medicines from consumers and responsible for post-registration observation of safety of medicine: AVVA Kazakhstan LLP, Republic of Kazakhstan 050057, Almaty, Bukhar Zhyrau St., 66/120, office 101-2. Ph. 8 (727) 274-11-17. e-mail:
To Develop info@avva-rus.ru
Additional information
| Ingredient |
|---|




















Reviews
There are no reviews yet.