Description
The instruction for medical use
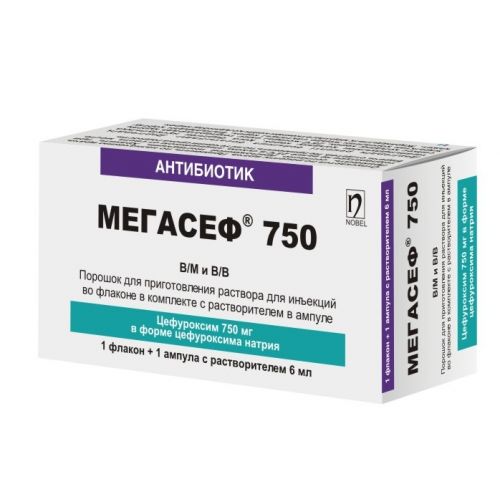
of medicine МЕГАСЕФ® 250 МЕГАСЕФ® 750
the Trade name
МЕГАСЕФ® 250 МЕГАСЕФ® 750
International unlicensed
name Tsefuroksim Lekarstvennaya a form
Powder for preparation of solution for injections of 250 mg and 750 mg complete with solvent (water for injections of 2 ml or 6 ml)
Structure
contains One bottle:
active agent – a tsefuroksima sodium salt of 250 mg or 750 mg,
One ampoule contains:
solvent – water for injections of 2 ml (for a dosage of 250 mg) or 6 ml (for a dosage of 750 mg)
the Description
White or whitish, slightly hygroscopic powder
Pharmacotherapeutic group
Antimicrobial drugs for system use. Beta laktamnye antibacterial drugs other. Cephalosporins of the second generation. Tsefuroksim.
ATX J01DC02 code
the Pharmacological
Pharmacokinetics Later properties of an intramuscular injection of 750 mg of a tsefuroksim average peak concentration in serum (Cmax) makes 27 mkg/ml. It reaches concentration in plasma (tmax) approximately in 45 minutes.
Elimination half-life (t1/2) after an intramuscular or intravenous injection makes about 80 minutes. About 50% of a tsefuroksim contact serum protein. Tsefuroksim is found in the volumes exceeding the maximum effective concentration in pleural liquid, intra articulate liquid, bile, saliva, bones and in liquid of chambers of the eye. About 89% of a dose of a tsefuroksim are allocated with kidneys without changes during 8 h that results in high concentrations in urine. Tsefuroksim gets through a blood-brain barrier in case of meningitis.
A pharmacodynamics
of Megasef® – a tsefalosporinovy antibiotic of the second generation of a broad spectrum of activity. Has bactericidal effect at the expense of inhibition of synthesis of a cell wall of bacteria. Мегасеф® acetylates membrane-bound transpeptidases, breaking, thus, the cross stitching of peptido-glycanes necessary for ensuring durability and rigidity of a cell wall. Мегасеф® it is resistant to action of many bacterial beta laktamaz therefore it is active concerning the majority of the types resistant to penicillin and amoxicillin.
Мегасеф® it is active concerning the following microorganisms:
Gram-negative bacteria: Escherichia coli, Haemophilus influenzae (including the types producing beta lactamelements), Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis (including the types producing beta lactamelements), Neisseria gonorrhoeae (including the types producing beta lactamelements), Salmonella spp., Shigella spp., Proteus mirabilis, Enterobacter spp.
Gram-positive bacteria: Staphylococcus aureus (including the types producing beta lactamelements), Streptococcus pneumoniae, Streptococcus pyogenes.
Certain types of enterococci, for example Enterococcus faecalis, are resistant to a tsefuroksim.
Anaerobe bacterias: Peptococcus spp., Peptostreptococcus spp., Clostridium spp., Bacteroides spp., Fusobacterium spp.
Indications
– an acute bronchitis, exacerbation of chronic bronchitis, bacterial pneumonia, abtsess lungs
– otitis, sinusitis, tonsillitis, pharyngitis
– acute and chronic pyelonephritis, cystitis, an urethritis, a symptomless
bacteriuria
– infections of skin and soft tissues, including erysipelatous inflammation, wound fevers
– osteomyelitis, septic arthritis
– inflammatory diseases of bodies of a small pelvis
– gonorrhea
– peritonitis
– a septicaemia
– meningitis
– an endocarditis
– prevention of infectious complications at abdominal organs surgeries, a basin, heart, lungs, a gullet and vessels, at the orthopedic
operations Route of administration and doses
Drug is intended for intramuscular and intravenous administration.
Adults
In infections of moderate severity 3 times a day are recommended intramusculary or intravenously on 750 mg.
In heavy infections – intravenously on 1.5 g 3 times a day. If necessary the interval between injections can be reduced to 6 h, a daily dose of drug – 3 – 6 g. The course of treatment makes 5 – 10 days.
In some infections the use of drug on 0.75-1 g 2 times a day is more effective. The course of treatment makes 5 – 10 days. Further use of the tableted Megasef® form is possible.
Children are more senior than 3 months
the Recommended dose of drug of 30-100 mg/kg a day in 3-4 introductions. For the majority of infections the optimum daily dose makes 60 mg/kg.
The newborn appoint 30-100 mg/kg a day in 2-3 introductions.
Pneumonia
In pneumonia 2 times a day (intramusculary or intravenously) during 48-72 h recommend to administer the drug on 1.5 g. Further use of the tableted
form MEGASEF® Gonorrhoea At to gonorrhea drug is possible appoint in a dose 1.5 g once in the form of the 1st injection or in the form of 2 injections on 750 mg.
Meningitis
In meningitis the adult is appointed intravenously on 3 g by 3 times a day. To children 3 months are more senior appoint intravenously 150-250 mg/kg a day in 3-4 introductions. The newborn appoint drug intravenously in a dose of 100 mg/kg a day.
For prevention of infectious complications at abdominal, pelvic and orthopedic operations of drug enter intravenously in a dose 1.5 g along with introduction anesthesia. If necessary additional administrations of drug intramusculary in a dose of 750 mg in 8 and 16 h are possible. At heart surgeries, lungs, a gullet and vessels enter 1.5 g of drug intravenously with anesthesia induction, then enter 750 mg 3 times a day for the subsequent 24-48 h.
The renal failure
In a renal failure has to be applied a reduced dose. The dosage has to be defined by extent of renal disturbances and sensitivity of a microorganism.
A dosage of Megasef® for injections the adult with a renal failure:
Clearance of creatinine (ml/min.)
the Dose (mg)
the Frequency
& gt,
20 750 – 1500
Every 8th
Parts 10 –
20,750
Each 12 h
& lt,
10,750
Each 24 h
Patients with a renal failure which is on continuous a hemodialysis with use of the arteriovenous shunt or on haemo filtration of high speed in intensive care units, Megasef®. appoint in a dose 750 g 2 times a day. For the patients who are on haemo filtration of low speed appoint the doses recommended in a renal failure.
Side effects
Side reactions happen very seldom and have passing character.
Undesirable reactions differ on frequency according to the following classification: very often ≥1/10, it is frequent ≥1/100 – & lt, 1/10, infrequently ≥1/1000 – & lt, 1/100, is rare ≥1/10000 – & lt, 1/1000, also lt, 1/10000 is very rare.
Often
– a neutropenia, an eosinophilia
– tranzitorny increase in liver enzymes
– reactions in the place of an injection (pain, thrombophlebitis)
Bol after intramuscular introduction arises at introduction of high doses more often, nevertheless it should not serve as the reason for treatment cancellation.
Infrequently
– a leukopenia, decrease in concentration of hemoglobin, the false positive test of Koombs
– skin rash, a small tortoiseshell, an itching
– digestive tract disorder
– tranzitorny increase in level of bilirubin (especially at patients with the previous liver pathology)
Is rare
– the increased growth of mushrooms of the sort Candida
– thrombocytopenia
– fever of a medicinal etiology
Very seldom
– hemolytic anemia
Cephalosporins belong to the class of antibiotics which are adsorbed on a surface of membranes of erythrocytes and interact with the antibodies directed to this medicinal substance that can lead to false positive reaction of Koombs or hemolytic anemia.
– intestinal nephrite
– an anaphylaxis
– a skin vasculitis
– pseudomembranous colitis
– an exudative multiformny erythema, Stephens-Johnson’s syndrome, a toxic epidermal necrolysis
– increase in level of creatinine, urea nitrogen and decrease in clearance of creatinine
of the Contraindication
– hypersensitivity to a tsefuroksim and other cephalosporins
– pregnancy and the period of a lactation
– bleeding and a disease of digestive tract
Medicinal interactions
Is recommended use of a method of a glyukozoksidaza or hexokinase when determining level of glucose in blood plasma at the patients receiving tsefuroksy. False positive reactions to presence of glucose in urine in tests with copper restoration can take place (Benedikta, Fekhlinga, Klinitest). For definition of a glucosuria it is necessary to use fermental tests. Nevertheless, it does not cause false positive results as some other cephalosporins. Positive forward reaction of Koombs is also possible.
Tsefuroksim, suppressing indestinal flora, interferes with vitamin K synthesis. Therefore at simultaneous use with the drugs reducing aggregation of thrombocytes (non-steroidal anti-inflammatory drugs, salicylates, Sulfinpyrazonum) the risk of developing bleedings increases. For the same reason at simultaneous use with anticoagulants strengthening of anticoagulating action is noted. At simultaneous use with loopback diuretics the risk of development of nephrotoxic action increases.
Co-administration of a probenetsid with tsefuroksimy slows down canalicular secretion, increases peak concentration in serum approximately by 40% and increases elimination half-life approximately by 30%.
Special instructions
At patients with hypersensitivity to penicillin are possible allergic reactions to antibiotics of group of cephalosporins, such patients should appoint cephalosporins with care.
High doses of cephalosporins have to be appointed with care in the profound renal failures and also to the patients receiving the accompanying treatment by strong diuretics as furosemide and aminoglycosides.
Patients with digestive tract diseases (especially in colitis) should apply with care.
At emergence of allergic reaction it is necessary to stop administration of drug.
Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms
Drug does not affect ability to run the vehicle and work with potentially dangerous mechanisms.
Overdose
Symptoms – spasms.
Treatment: hemodialysis and peritoneal dialysis.
The form of release and packing
place Drug in the bottle from colourless glass corked by a rubber bung and which is pressed out by an aluminum cap with a protective plastic cover. On a bottle paste the self-adhesive label.
Solvent is placed in ampoules from colourless glass. Apply the text on an ampoule with method of an intaglio printing the fast fixed paint.
On 1 bottle with drug and 1 ampoule with solvent of 2 ml (for a dosage of 250 mg) or of 6 ml (for a dosage of 750 mg) together with the instruction for medical use in the state and Russian languages place in a cardboard pack.
To Store storage conditions in the dry, protected from light place, at a temperature not above 25 °C.
To store out of children’s reach!
3 years
not to apply a period of storage after an expiration date.
Prescription status
According to the prescription
the Producer Nobel Ilach Sanai ve A.Sh. Tidzharet, Turkey
the Packer
of JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika
Republic of Kazakhstan Almaty, Shevchenko St. 162 E.
The owner of the registration certificate
of JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika
the Republic of Kazakhstan
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods):
JSC Nobel Almatinskaya Pharmatsevticheskaya Fabrika
Republic of Kazakhstan Almaty, Shevchenko St. 162 E.
Phone number: (+7 727) 399 – 50 – 50
Fax number: (+7 727) 399 – 60 – 60
To develop the e-mail address of nobel@nobel-aff.kz
Additional information
| Ingredient |
|---|

















Reviews
There are no reviews yet.