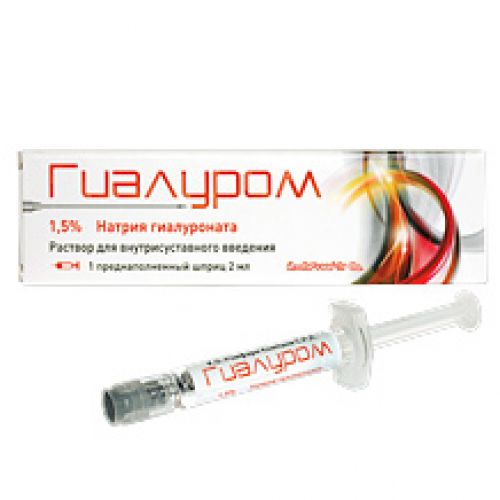
Gialurom 30 mg / 2 ml 1’s solution for intraarticular administration in the syringe
$155.00
Description
The name of a product of medical purpose
by Gialur, solution for intra articulate introduction of 30 mg / 2 ml, the prefilled syringe of 2 ml No. 1,
the No. 3 Structure and the description
of the product The Prefilled Syringe with a capacity of 2.25 ml from not less than 2 ml of solution for intra articulate introduction, No. 1, No. 3.
1 ml of solution contains sodium hyalrunate of 15 mg, sodium chloride of 9 mg and water for injections to 1 ml.
Sodium hyalrunate is received as a result of fermentation.
Gialur is issued in the form of the prefilled syringes from transparent glass of hydrolytic class I which contain not less than 2 ml of sterile, vyazkoelastichny solution. On each prefilled syringe paste the label. On 1 prefilled syringe place in strip plastic packaging with a covering from aluminum foil. On 1 or 3 strip plastic packagings with equal quantity of sterile needles of 21 in size G x 1 1/2’ ’(0.8 x 40) together with the instruction for medical use in the state and Russian languages in a pack from cardboard.
Name and (or) trademark
of the Rompharm Company manufacturing organization of S.R. L.
The scope
is applied by Gialur as vyazkoelastichny additive or to substitution of synovial fluid in joints of the person.
By Gialur it is applied in the pains caused by degenerative diseases of synovial joints, such as osteoarthritis. Effect of the drug Gialurom is lubricant and mechanical support.
Sodium hyalrunate represents polysaccharide, consists of the repeating disakharidny links of N-atsetilglikozamina and sodium glyukuronat, with a molecular weight up to 2400000 Yes.
Sodium hyalrunate is present everywhere at a human body and is present at high concentrations in fabrics, such as vitreous, synovial fluid, an umbilical cord and a derma. In synovial fluid of sodium hyalrunate acts as lubricant and the absorber of blow that the normal movement without pain allows.
In an arthritis type disease, the viscoelasticity of synovial fluid decreases so considerably increases mechanical load of a joint and destruction of an articulate cartilage amplifies, it is shown by restriction and oxycinesias in joints.
The greasing and shock-absorbing effect of this drug alleviates pains and improves mobility of a joint at intra articulate use. These effects proceed more than 6 months after one course of treatment.
The Injection route of administration Gialur are made by specialists doctors.
By Gialur it is intended only for intra articulate use.
Do not apply intravenously.
The entered volume depends on the joint size, but does not exceed 2 ml in a knee joint and other big joints, or 1 ml for small joints. The doctor is responsible behind establishment of applicable volume and has to be convinced that the joint is not rebooted.
By Gialur it is entered in a joint cavity once a week within 3 weeks in a row. Perhaps simultaneous treatment of several joints. The recycle of treatment of the same joint cannot be carried out before 6 months.
Savings of liquid in a joint have to be removed by absorption before use by Gialur.
Gialur is issued in the form of the prefilled syringe which contents do not need to be diluted. Contents of the prefilled syringe Gialurom are sterile and it is necessary to use right after packing opening.
By Gialur it has to be entered accurately into a joint cavity, carefully observing the scheme of introduction and the rule of an asepsis and antiseptics at intra articulate introduction.
The ready syringe is removed from sterile strip plastic packaging, the cap is removed then the sterile needle which is fixed by small turn is put on. Before introduction it is necessary to remove air from the syringe.
Data necessary for the user for identification of a product of medical purpose
Completeness
No.
Components
manufacturing Organization (manufacturer)
Country
1.
The prefilled syringe with a capacity of 2.25 ml from 2 ml of solution for intra articulate introduction, No. 1, No. 3.
1 ml of solution contains sodium hyalrunate of 15 mg, sodium chloride of 9 mg and water for injections to 1 ml.
The syringe with a capacity of 2.25 ml consists from:
Bodies from colourless glass
of the Piston
of the Rod of the piston
of the Emphasis for fingers
BD Medical Pharmaceutical Systems
France
2.
Syringe needle 21 G x 1 1/2’ ’(0.8 x 40)
CHIRANA T. Injecta a.s.
Slovakia
Storage conditions
At a temperature not higher than 25 of 0C, in original packing.
Not to freeze.
To store out of children’s reach.
3 years
not to apply an expiration date after expiry date.
Gialur in the form of the prefilled syringes for single use contains not less than 2 ml of sterile, vyazkoelastichny solution.
Use of the opened and/or damaged sterile packing is not allowed. Repeated sterilization is not allowed.
By Gialur it is intended only for single use.
Reuse is not allowed.
The Rompharm Company manufacturing organization of S.R. L., Romania
Side effect
Is possible emergence of the local secondary phenomena, such as pains, caumesthesia, reddening and swelling.
At intra articulate introduction there are minimal risks connected with an infection and bleeding.
Contraindications for use
· individual intolerance (including hypersensitivity in the anamnesis) to the Gialurom components
· presence of contaminated wounds, grazes in a joint
· infectious diseases of joints
· known system disturbances of blood clotting.
Gialur may contain traces of bacterial proteins and it is contraindicated to patients with the corresponding hypersensitivity.
Precautionary measures (safety)
It is necessary to observe the scheme of introduction and the rule of an asepsis and antiseptics at intra articulate introduction.
Injections Gialur have to be made by exclusively diplomaed and certified doctors of the corresponding profile.
Not to use Gialur in excess quantity and it is necessary to watch patients carefully. Not to overload interarticular space.
Before use by Gialur the patients have to be carefully examined for detection of signs of an acute inflammation, and the doctor has to solve if it is necessary to begin treatment with sodium with hyalrunate in that case.
Till today do not exist sufficient data to recommend use at children and teenagers.
It is not necessary to use Gialur at the same time or in mix with other drugs intended for intra articulate use.
The drug is administered only if solution is transparent.
Introduction by Gialur is carried out at the room temperature.
Measures of first-aid treatment at misuse or collateral influence.
As well as after any other invasive procedure on a joint, after an injection Gialur, the patient is recommended to adhere to the sparing mode and to avoid excessive load of a joint within several days.
Patients with painful consequences after intra articulate introduction
by Gialur should see a doctor immediately. If pain amplifies during introduction, the procedure has to be stopped.
Imposing of ice on a punktirovanny joint within 5-10 minutes allows to reduce the frequency of emergence of the similar phenomena.













Reviews
There are no reviews yet.