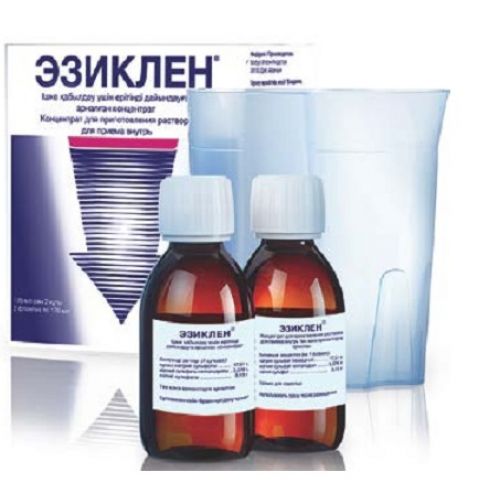
Eziklen® 2’s 176 ml conc. for a solution for pr. int.
$41.50
Description
The instruction for medical use of Eziklen Torgovoye medicine a name Eziklen Mezhdunarodnoye the unlicensed name Is not present the Dosage form the Concentrate for preparation of solution for intake Structure One bottle contains active agents: sodium sulfate anhydrous – 17.510 g, magnesium sulfate heptahydrate – 3.276 g, potassium sulfate – 3.130 g excipients: Natrium benzoicum (E211), anhydrous citric acid, malic acid, sucralose, fragrance fruit cocktail * * composition of fragrance: natural and synthetic fragrances, propylene glycol (E1520), alcohol, acetic acid, benzoic acid (E210) the Description Transparent or slightly muddy liquid with a smell of mix of fruit Pharmacotherapeutic group Laxatives. Osmotic laxatives. The combination of mineral salts the ATX A06AD10 Code the Pharmacological Pharmacokinetics Absorption properties of sulfate represents limited and saturable process of active transport. The absorbed sulfate is excreted mainly by kidneys. In clinical trials after reception of the structure similar in the content of sulfates to the drug Eziklen, at six healthy volunteers (in the mode of fractional use, i.e. reception of two doses with a break in 12 h) the maximum concentration of sulfate in serum was observed approximately in 16 h after reception of the first dose and in 5 h after reception of the second dose [C max: 499.50 µmol/l (CV: 33.03%), in comparison with a reference value of 141 – 467 µmol/l, on average 335 µmol/l (CV: 34.40%)]. Then serumal concentration of sulfates decreased, and elimination half-life made 8.5 h (CV: 53.76%). Excretion with a stake was the main way of elimination of sulfates from an organism (about 70% of the accepted quantity). System influence (AUC and Cmax) the drug Eziklen on the level of sulfates was studied during the comparative research at healthy volunteers, at 6 patients with a moderate renal failure (clearance of creatinine from 30 to 49 ml/min.) and at 6 patients with a slight or moderate liver failure (the classes A (N=5) and B (N=1) on Child-Pugh scale, respectively). The renal failure led to decrease in amount of the sulfates removed with urine. Therefore, average AUC and Cmax values were about 50% higher at patients with a renal failure in comparison with healthy volunteers. System impact of drug on the level of sulfates did not depend on a liver failure. In all three studied groups the concentration of sulfates in serum returned to a reference value for the 6th day after administration of drug of Eziklen. In this research the administration of drug of Eziklen did not lead to clinically significant increase in level of sulfates in blood at patients with a liver or renal failure. Eziklen’s pharmacodynamics is osmotic depletive. The mechanism of effect of drug first of all is caused by limited and saturable process of active transport of sulfate. At achievement of a limit of saturation of absorption, sulfates remain in an intestines gleam. The osmotic effect of not absorbed sulfate causes a water delay in a gleam of intestines and leads to cleaning of intestines. The osmotic effect of not absorbed ions at intake of large volume of water causes plentiful watery diarrhea. In clinical trials the average time before appearance of the profound diarrhea was about 6.3 h at the mode of fractional use (reception of two doses at an interval of 12 h) and about 2.8 h at the mode of single use (reception of two doses at an interval of 1 h). Indications – purgation at adult patients before holding any medical procedure demanding lack of contents in intestines (for example, visualization of intestines at an endoscopic and radiological research or before surgical interventions). Route of administration and doses Inside. Appropriate purgation requires reception of two bottles of the drug Eziklen. Before reception the contents of each bottle are parted with water by means of the enclosed cup to the total amount of a cup by about 0.5 l. After opening of a bottle and/or cultivation by water drug has to be used immediately. Within the next two hours the patient has to drink the received divorced solution and in addition drink about 1 l (2 cups filled to a tag) of water or transparent liquid. The resolved transparent liquids are: water, tea or coffee (without milk, cream or not milk substitutes of cream), carbonated or not carbonated soft drinks, the filtered fruit juice without pulp (except juice of red or violet color), transparent broths or soups filtered from solid ingredients. In total, the volume of the liquid accepted inside necessary for cleaning of intestines before holding a procedure is about 3 liters. The drug can be taken or in the mode of fractional use (reception in two days: the first bottle is accepted on the eve of the procedure in the evening, and the second – the next morning), or in the mode of single use (reception in one day). The suitable mode of drug dosing Eziklen can be defined by the doctor. If time of the appointed procedure allows, then the mode of fractional use is more preferable, than the mode of one-time reception in one day. The mode of one-time reception in one day is potentially possible alternative mode of use of drug. The Mode of fractional use (reception in two days) Put a use method on the eve of the procedure: On the eve of the procedure (for example, at 18:00) the following iinstruktion need to follow: • Contents of one bottle of the drug Eziklen should be poured out in the enclosed cup and to part with water to a tag (i.e. up to the volume about 0.5 l). • The patient has to drink the received solution, then within the next two hours it is necessary to drink in addition two cups of water or transparent liquid filled to a tag (i.e. about 1 l). Day of the procedure: In the morning in day of the procedure (in 10-12 h after evening administration of drug) it is necessary actions, according to iinstruktion on reception of an evening dose: • Contents of the second bottle of the drug Eziklen should be poured out in the enclosed cup and to part with water to a tag (i.e. up to the volume about 0.5 l). • The patient has to drink the received solution, then within the next two hours it is necessary to drink in addition two cups of water or transparent liquid filled to a tag (i.e. about 1 l). Reception of full volume of divorced solution of the drug Eziklen and an additional amount of water or transparent liquid has to be complete at least in 1 hour prior to the procedure. The mode of single use (reception in one day) (the alternative mode of dosing for use depending on individual clinical need of the patient) Evening on the eve of the procedure: On the eve of the procedure (for example, at 18:00): • Contents of one bottle of the drug Eziklen should be poured out in the enclosed cup and to part with water to a tag (i.e. up to the volume about 0.5 l). • The patient has to drink the received solution, then within the next two hours it is necessary to drink in addition two cups of water or transparent liquid filled to a tag (i.e. about 1 l). Approximately in 2 h after the beginning of reception of the first dose (for example, at 20:00): • Contents of the second bottle of the drug Eziklen should be poured out in the enclosed cup and to part with water to a tag (i.e. up to the volume about 0.5 l). • The patient has to drink the received solution, then within the next two hours it is necessary to drink in addition two cups of water or transparent liquid filled to a tag (i.e. about 1 l). Reception of full volume of divorced solution of the drug Eziklen and an additional amount of water or transparent liquid has to be finished at least in 1 hour prior to the procedure. Open for IInstruktsiya on use of drug a bottle, having pressed a cover and turning it counterclockwise. Pour contents of one bottle in the enclosed cup Add in a cup water to a tag Drink all liquid from a cup slowly, within 30-60 minutes IMPORTANT: Drink two (2) more cups of water or transparent liquid. Every time fill a cup to a tag Drink all liquid from each cup slowly, within not less than 30 minutes the Performance of stages 1-6 takes about 2 hours. It is necessary to repeat all stages with the second bottle of the drug Eziklen. After the procedure For completion of loss of liquid by preparation for the procedure, patients have to drink enough liquid to support appropriate level of hydration. Dietary restrictions A day before holding a procedure the light breakfast can be used. Then for lunch, a dinner and during other meals up to the moment of holding a procedure it is necessary to use only transparent liquids. It is necessary to avoid intake of liquids of red and violet color, milk and alcoholic beverages. Special groups of patients Elderly patients the difference in efficiency and safety of the drug Eziklen at elderly patients and patients of other age groups was Not revealed during clinical development of drug. Elderly patients do not require correction of a dosage, however for such patients it is necessary to take special precautionary measures, as for group of high risk. Patients with a renal failure Exist limited data on this group of patients. Correction of a dose for patients with a renal failure from easy to moderate severity is not required, however, it is necessary to take special precautionary measures for these patients as group of high risk. The drug Eziklen should not be used at patients with heavy degree of a renal failure. Patients with a liver failure Exist limited data on this group of patients. Correction of a dose for patients with a liver failure is not required, however, it is necessary to take special precautionary measures for these patients as group of high risk. Side effects Diarrhea is the expected result of preparation of intestines by means of cleaning therefore this effect Eziklen takes place after administration of drug. As well as in a case with any intervention of this kind, side effects appear at most of patients. The most frequent reported side reactions in clinical trials and post-registration observation were: discomfort, abdominal distension, abdominal pain, nausea and vomiting. During clinical trials when using the mode of single use (reception in one day) messages about vomiting arrived with a bigger frequency, than when using the mode of fractional use. Frequency of side reactions against the background of administration of drug of Eziklen is classified as follows: Very often (≥1/10), it is frequent (≥1/100 – & lt, 1/10), infrequently (≥1/1000 – & lt, 1/100), is rare (≥1/10000 – & lt, 1/1000), is very rare (& lt, 1/10000), it is unknown (it cannot be estimated on the basis of available data). System body class Frequency Side medicinal reaction of Disturbance from the immune system It is unknown (post-registration data) Hypersensitivity (including urticaria, an itching, rash, an erythema, dispnoe, feeling of “lump” in a throat) Disturbances from nervous system Infrequently the Disturbance Headache from digestive tract the Abdominal distension, abdominal pain, nausea, vomiting Infrequently Discomfort in anorectal area, dryness in the Disturbance mouth Is very frequent from kidneys and urinary tract Infrequently the Dysuria of Disorder of the general character and reaction in the injection site Very often Discomfort Infrequently the Fever Laboratory and tool data Infrequently Increase in level aspartate – aminotransferases, increase in level of a kreatinfosfokinaza in blood, increase in level of a lactate dehydrogenase in blood, increase in level of phosphorus in blood, a hyperbilirubinemia, deviations in results of biochemical analysis of blood, including a hyponatremia, a hypopotassemia, a hypocalcemia and a hyperuricemia Additional information for special groups of patients during clinical trials the passing increase in level of uric acid was observed. For patients who in the anamnesis have clinical manifestations of gout or a hyperuricemia see the section “Special Instructions”. During clinical trials the difference in safety of administration of drug Eziklen at elderly patients and patients of other age groups was not revealed. However for elderly patients as group of high risk, it is necessary to take special precautionary measures. Contraindications – hypersensitivity to active agents or any components of drug – gastrointestinal obstruction or suspicion on gastrointestinal obstruction – perforation of intestines – gastric emptying disturbance (for example, gastroparez) – impassability of intestines (Ilheus) – toxic colitis or toxic megacolon – profuse vomiting – dehydration of heavy degree – stagnant heart failure – ascites – a heavy renal failure (KF & lt, 30 ml/min. / 1.73 sq.m) – inflammatory bowel diseases in an active phase (for example, Crohn’s disease, ulcer colitis) – pregnancy, the breastfeeding period – children’s age up to 18 years Medicinal interactions As well as for any other drugs for cleaning of intestines: – With care to apply at the patients receiving therapy by the drugs influencing the level of electrolytes: blockers of calcium channels, diuretics, drugs of lithium or other drugs of similar action. – It is necessary to be careful at a concomitant use of drugs which, as we know, extend QT interval. – The expected result of use of Eziklen is diarrhea, and simultaneous oral administration of other medicines within 1 – 3 hours after the beginning of administration of drug and up to completion of process of cleaning of intestines can bring to washing away from digestive tract or to insufficient absorption of at the same time applied medicines. In particular, it can be influenced therapeutic effect of regularly taken oral drugs with the narrow therapeutic index or short elimination half-life (for example, oral contraceptives, antiepileptic drugs, antidiabetic drugs, antibiotics, left thyroxine, digoxin, etc.). Special instructions Electrolytic disorders and dehydration: – Considering potential risk of heavy electrolytic disorders, it is necessary to consider carefully a ratio advantage/risk before using the drug Eziklen at patients from groups of high risk. Before prescribing of drug contraindications for its appointment have to be excluded. Special attention needs to be paid to use of special precautionary measures, including importance of maintenance of adequate hydration. – All patients have to be warned about need of maintenance of adequate hydration to, in time and after administration of drug Eziklen. If at the patient the plentiful vomiting or symptoms of dehydration after administration of drug develops, measures for rehydration in order to avoid risk of the serious complications connected with disturbance of water and electrolytic balance (such as spasms and cardiac arrhythmia) have to be taken. Besides, it is necessary to take into account a possibility of carrying out laboratory analyses before the planned procedure (electrolytes, creatinine and urea nitrogen in blood). Patients need to recommend to use such additional amount of water or transparent liquids which can be required for maintenance of appropriate level of hydration. Patients from risk groups: – For the weakened exhausted patients, patients of advanced age, patients with clinically significant renal failures, a liver or heart and also for patients with risk of development of electrolytic disturbances the doctor has to consider the possibility of a research on electrolytes and conducting functional renal tests before drug use. – Dehydration or electrolytic disturbances have to be corrected prior to use of drug for cleaning of intestines. Besides, it is necessary to observe extra care when using drug at patients with morbid conditions or at the patients accepting medicines which increase risk of emergence of disturbances of water and electrolytic balance (including a hyponatremia and a hypopotassemia) as in this case the risk of potential complications can increase. For these patients it is necessary to carry out nadl
zhashchy monitoring. – There is a theoretical risk of lengthening of an interval of QT which can appear owing to an electrolytic imbalance. To use drug with care at patients: – With disturbance of an emetic reflex and at the patients predisposed to regurgitation or aspiration. Such patients have to be under observation during use of drug for cleaning of intestines. – With disorders connected with the lowered motility of digestive tract or at patients who have in the anamnesis of a state or surgical interventions on digestive tract which resulted in predisposition to a digestive tract hypokinesia. Hyperuricemia: – Administration of drug Eziklen can cause a passing lung or moderate increase in content of uric acid. The possibility of increase in level of uric acid has to be considered before prescribing of drug at patients who in the anamnesis have gouty manifestations or a hyperuricemia. Additional information: – Eziklen is not intended for reception without preliminary cultivation of a concentrate. Use of undiluted solution can increase risk of nausea, vomiting, dehydration and electrolytic disorders. Each bottle of drug needs to be parted with water and also to use the additional recommended amount of water for ensuring tolerance of drug with the patient. – This medicine contains 247.2 mmol (or 5.683 g) sodium in 1 bottle. This fact needs to be considered concerning patients who keep to a diet with controlled consumption of sodium. – This medicine contains 35.9 mmol (or 1.405 g) potassium in 1 bottle. This fact needs to be considered concerning patients with reduced function of kidneys or patients who keep to a diet with controlled consumption of potassium. Features of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms the Overdose in case of overdose or the wrong use does not influence (for example, intake not of divorced drug and/or the use of insufficient volume of water), developing of nausea, vomiting, diarrhea and electrolytic disturbances is possible. Conservative measures usually are sufficient, it is necessary to perform oral rehydration therapy. In rare instances the overdose causes the heavy metabolic disturbances demanding use of intravenous rehydration. The form of release and packing place About 176 ml of a concentrate in a bottle from polyethyleneterephthalate of amber color with a capacity of 180 ml with the screwing-up cover from polyethylene of the high density protected from accidental opening by children. Two bottles with a concentrate and one polypropylene cup about 500 ml for cultivation and administration of drug together with the instruction for medical use in the state and Russian languages place in a pack from cardboard. To Store storage conditions at a temperature not above 30 °C to Store in original packing for protection against light. To store out of children’s reach! Period of storage 3 years. After opening of a bottle and/or cultivation by water drug has to be used immediately. Not to use after the expiration date specified on packing. Prescription status According to the prescription the Producer Bofur Ipsen Indastri, France the Owner of the registration certificate Ipsen of Pharm, France the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods): Representative office of JSC IPSEN PHARMA (IPSEN FARMA) in RK 050040 Almaty, Al-Farabi Ave., 45, office 2 Ph./fax: 8 (727) 2646448, 2646620, 2646715
To develop



















Reviews
There are no reviews yet.