Description
The instruction on medical
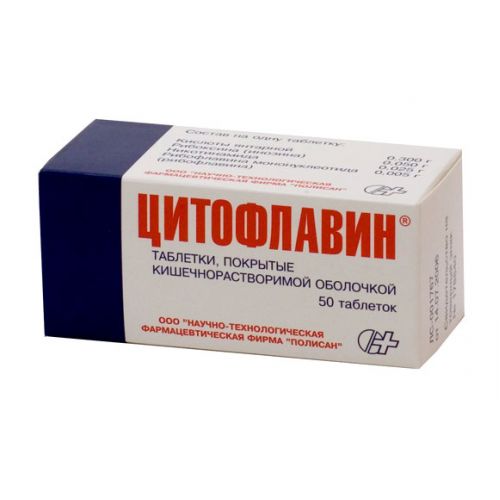
Trade name
ЦИТОФЛАВИН®
the International unlicensed name
Is not present primeneniyulekarstvenny means ЦИТОФЛАВИН®
the Dosage form
of the Tablet, covered with a kishechnorastvorimy cover
Structure
One tablet contains
active agents:
succinic acid – 0.300 g,
inosine (inosine) – 0.050 g,
niacinamide – 0.025 g,
Riboflavinum sodium phosphate (Riboflavinum) – 0.005 g,
excipients: povidone, calcium stearate, gipromelloza, polysorbate-80,
structure of a cover: methacrylic acid and ethyl acrylate copolymer, propylene glycol, an azoruby (E 122), tropeolin – the Lake.
The description
of the Tablet of round shape, with a biconvex surface, coated red color. On cross section two layers are visible. A kernel of tablets from yellow till yellow-orange color.
Pharmacotherapeutic group
Other drugs for treatment of diseases of nervous system.
The ATX N07XX code
the Pharmacological
Pharmacokinetics of Tsitoflavin properties has high bioavailability.
Succinic acid at intake gets from digestive tract into blood and fabrics, participating in reactions of energy balance, and completely breaks up to the final products of exchange (carbon dioxide and water) in 30 minutes.
Inosine is well absorbed from digestive tract. Time of achievement of the maximum concentration in blood – 5 hours, the average time of deduction in blood – 5.5 hours, the equilibrium volume of distribution is about 20 liters. Inosine is metabolized in a liver with formation of the inozinmonofosfat with the subsequent its oxidation to uric acid. In insignificant quantity it is removed by kidneys.
Niacinamide is quickly distributed in all fabrics (the equilibrium volume of distribution about 500 liters). Time of achievement of the maximum concentration in blood – 2 hours, the average time of deduction in blood – 4.5 hours. Niacinamide gets through a placenta and into breast milk, is metabolized in a liver with formation of N-metilnikotinamida, removed by kidneys.
Riboflavinum is quickly absorbed from digestive tract, distributed unevenly (the greatest number in a myocardium, a liver, kidneys), transformed in flavinadeninmononukleotid (FMN) and flavinadenindinukleotid (FAD) in mitochondrions. Gets through a placenta and into breast milk, it is removed by kidneys, mainly in the form of metabolites.
A pharmacodynamics
Pharmacological effects are caused by combined effect of the components which are a part of the drug TsITOFLAVIN.
Succinic acid – the endogenous intracellular metabolite of a tricarbonic acid cycle performing the general power synthesizing function in organism cells.
With the participation of a coenzyme of a flavinadenindinukleotid (FAD), succinic acid mitochondrial enzyme is quickly transformed by succinatedehydrogenase to fumaric acid and further to other metabolites of a cycle of tricarboxylic acids. Stimulates aerobic glycolysis and synthesis of ATP in cells.
The final products of metabolism of succinic acid in a tricarbonic acid cycle are carbon dioxide and water. Succinic acid improves tissue respiration due to activation of transport of electrons in mitochondrions.
Riboflavinum (B2 vitamin) is a flavin coenzyme (FAD) activating succinatedehydrogenase and other redoxreactions of a tricarbonic acid cycle.
Niacinamide (RR vitamin), niacin amide. Niacinamide in cells by a cascade of biochemical reactions is transformed to a form of a nikotinamidadeninnukleotid (OVER) and its phosphate (NADF), activating niacinamide – the dependent enzymes of a tricarbonic acid cycle necessary for cellular respiration and stimulation of synthesis of ATP.
Inosine is derivative purine, the predecessor of ATP. Has ability to activate a number of enzymes of a tricarbonic acid cycle, stimulating synthesis of key enzymes nucleotides – a flavinadenindinukleotid (FAD) and nicotinamide adenine dinucleotide (NAD).
Thus, all TSITOFLAVIN®a components are natural metabolites of an organism and stimulate tissue respiration. The metabolic power correction, anti-hypoxemic and antioxidant activity of drug defining pharmacological properties and medical efficiency of components are caused by complementary effect of succinic acid, inosine, niacinamide and Riboflavinum.
Indications
At adults in complex therapy:
– brain heart attack consequences
– other cerebrovascular diseases (cerebral atherosclerosis, hypertensive encephalopathy)
– a neurasthenia (acrimony, fatigue, loss of ability to long intellectual and physical tension)
the Route of administration and doses
Inside on 2 tablets 2 times a day with an interval between receptions of 8-10 hours. It is necessary to take a pill not less than in 30 minutes prior to food, without chewing, washing down with water (100 ml).
Administration of drug in morning and day time of day is recommended (no later than 18 hours).
Duration of a course of treatment – 25 days. Purpose of a repeated course is possible with an interval not less than 1 month.
Side effects
– a headache
– pains or discomfort in epigastric area
– are possible allergic reactions in the form of skin hyperaemia and an itching.
The tranzitorny hypoglycemia, a hyperuricemia, exacerbation of the accompanying gout belong to undesirable reactions.
Contraindications
– individual intolerance of components of drug
– children’s age up to 18 years
Medicinal interactions
Succinic acid, inosine and niacinamide are compatible to other medicines. Riboflavinum reduces activity of some antibiotics (tetracyclines, erythromycin, lincomycin), is incompatible with streptomycin. Ethanol, tricyclic antidepressants, blockers of canalicular secretion reduce Riboflavinum absorption, and thyroid hormones accelerate his metabolism.
Special instructions
In a hypertension the correction of doses of hypotensive drugs can be required.
At patients with diabetes to carry out treatment under control of concentration of glucose to blood.
Perhaps intensive coloring of urine in yellow color.
Use during the periods of pregnancy and a lactation
is not recommended to be accepted at pregnancy and in the period of a lactation due to the lack of clinical data on efficiency and safety of drug during these periods.
The feature of influence of medicine on ability to run the vehicle and other potentially dangerous mechanisms
does not affect ability to run the vehicle and other potentially dangerous mechanisms.
Overdose
of Data on overdose of drug are absent.
A form of release and packing
On 10 tablets in blister strip packaging from a film of polyvinylchloride and aluminum foil for packing.
On 5 blister strip packagings together with the instruction for medical use in the state and Russian languages place in a pack from cardboard.
Storage conditions
In the place protected from light at a temperature not over 25 ºС.
To store out of children’s reach!
2 years
not to use a period of storage after the expiration date specified on packing.
Prescription status
According to the prescription
LLC Scientific and Technological Pharmaceutical Firm POLISANG Producer (LLC NTFF POLISANG).
Russian Federation, 192102, St. Petersburg, Salov St., 72, building 2A, ph./fax: (812) 710-82-25.
Owner of the registration certificate
of LLC Scientific and Technological Pharmaceutical Firm POLISANG (LLC NTFF POLISANG), Russian Federation.
The address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products (goods): Republic of Kazakhstan, Almaty, Bogenbay St. of the batyr, 136. Ph.: (7272) 72-57-51. E-mail: doctorkvi@gmail.com.


















Reviews
There are no reviews yet.