Description
The instruction for medical use
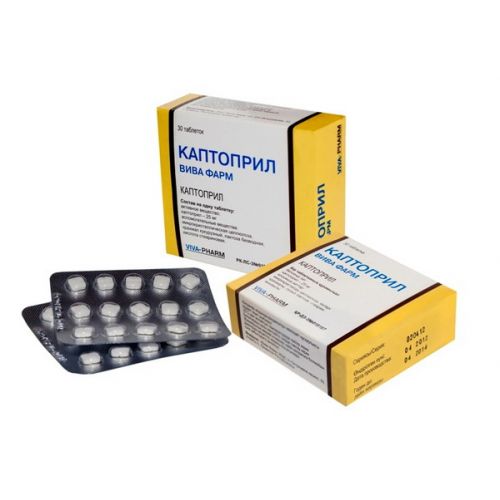
of VIVA FARM CAPTOPRIL medicine
the Trade name
VIVA FARM Captopril
the International unlicensed
name Captopril Dosage Form
of the Tablet, 25 mg
Structure
One tablet contains
active agent – captopril of 25 mg,
excipients: anhydrous lactose, corn starch, cellulose microcrystalline, stearic acid.
The description
of the Tablet of almost white color of square shape, biconvex, with slanted edges, crosswise risky on one party, with easy marbling.
Pharmacotherapeutic group
Drugs for treatment of diseases of a cardiovascular system.
The drugs influencing a system renin-angiotensin.
Angiotenzinkonvertiruyushchy enzyme inhibitors (AKF). Captopril.
The ATX C09AA01 code
the Pharmacological
Pharmacokinetics Later properties of intake, not less than 75% of drug is quickly soaked up in digestive tract. The maximum concentration of captopril in blood plasma is reached in 30-90 minutes. The concomitant use of food slows down absorption of drug for 30-40%. Antihypertensive action comes 20-30 minutes later after oral administration of captopril, the maximum action is reached 60-90 minutes later. Linking with proteins of blood plasma (mainly with albumine) makes 25-30%. Passes through gistogematichesky barriers, excepting a blood-brain barrier, through a placenta and gets into breast milk. It is metabolized in a liver, approximately for 50% in inaktivny disulfides (captopril disulfide and a captopril-cysteine-disulfide). Elimination half-life of not changed captopril makes about 2 hours, and connected – 12 hours. Within 24 hours, captopril is emitted with urine approximately for 95%, from them: 40-50% – in the form of not changed substance, the rest – in a look captopril disulfide and a captopril-cysteine-disulfide. In a renal failure the degree of discharge of captopril decreases. In this case it is necessary to reduce a dose or, respectively, to increase intervals between receptions.
The pharmacodynamics
VIVA FARM Captopril is antihypertensive drug, inhibitor of angiotenzinkonvertiruyushchy enzyme (AKF). The mechanism of antihypertensive effect of captopril is connected with competitive inhibition of activity of AKF that leads to reduction in the rate of transformation of angiotensin I into angiotensin II in fabrics and blood plasma owing to what vasodilating action is reached and there is a decrease in secretion of Aldosteronum in adrenal glands. As a result, captopril reduces the general peripheral vascular vascular resistance (afterload), jamming pressure in pulmonary capillaries (preloading) and resistance in pulmonary vessels, increases the minute volume of heart and tolerance to loading. At prolonged use, captopril reduces expressiveness of a hypertrophy of a myocardium of a left ventricle, prevents progressing of heart failure and slows down development of dilatation of a left ventricle. Expands arteries more than veins. Improves blood supply of an ischemic myocardium. Reduces aggregation of thrombocytes. Lowers a tone of the taking-out arterioles of balls of kidneys, thereby improving an intraglomerular hemodynamics, and interferes with development of a diabetic nephropathy.
Thanks to decrease in secretion of Aldosteronum in adrenal glands, the diuresis and a natriuresis amplifies, potassium level in blood serum increases, water exchange is normalized. Thereby, captopril promotes decrease in content of sodium at patients with chronic heart failure. The lack of negative return influence of angiotensin II on secretion of renin promotes increase in activity of renin in blood plasma. AKF leads Ingibition to increase in activity of the local systems kallikrein-kinina interfering bradykinin destruction (vasodepressor peptide) that leads to activation of a prostaglandinovy system. This mechanism also takes part in hypotensive effect of AKF inhibitors.
At the recommended daily dose the antihypertensive effect of captopril remains in the course of long therapy. The short-term termination of intake of captopril does not lead to fast, excessive increase in arterial blood pressure.
Indications
– arterial hypertension (including renovascular)
– chronic heart failure (as a part of complex therapy)
– dysfunction of a left ventricle after the postponed myocardial infarction at clinically stable state
– a diabetic nephropathy in insulin-dependent diabetes mellitus (at an albuminuria more than 30 mg/days)
the Route of administration and doses
Arterial hypertension
VIVA FARM Captopril should be accepted in the smallest effective dose which is selected individually for each patient. Each tablet of VIVA FARM Captopril has crosswise to risk on one party for division of a tablet (1 tablet contains 25 mg, ½ tablets – 12.5 mg, ¼ tablets – 6.25 mg of captopril).
The initial dose in easy/moderate arterial hypertension makes 12.5 mg (½ tablets) twice a day. The maintenance dose makes 25 mg (1 tablet) twice a day. If necessary the dose is increased by each 2-4 weeks. The maximum daily dose makes 150 mg.
In heavy arterial hypertension VIVA FARM Captopril is often combined with other hypotensive drugs, most often with thiazide diuretics (Hydrochlorthiazidum – on 25-50 mg/days). The dose of diuretic can raise each 1-2 weeks before achievement of the maximum dose which is applied at treatment of arterial hypertension.
Heart failure
Treatment by VIVA FARM Captopril should be begun under observation of the doctor. At use of an initial dose of 6.25 mg (¼ tablets VIVA FARM Captopril) three times a day it is possible to weaken effect of tranzitorny hypotension as much as possible. If necessary perhaps gradual (each two weeks) increase in a dose as much as possible up to 25 mg 2-3 times a day. In some cases the maximum daily dose can make 150 mg.
Dysfunction of a left ventricle after the postponed myocardial infarction at the patients who are in clinically stable state
Use of the drug VIVA FARM Captopril can be begun in
3 days after a myocardial infarction. The initial dose makes 6.25 mg/days (¼ tablets VIVA FARM Captopril), then the daily dose can be increased within several weeks to the level of 25 mg rub once a day (depending on tolerance of drug). If necessary a dose to the maximum daily dose three times a day gradually increase 50 mg. At development of arterial hypotension the dose decline can be required. The subsequent attempts of use of the maximum daily dose of 150 mg have to be based on tolerance of drug patients. Captopril can be applied together with other drugs, for example, of a trombolitikama, acetylsalicylic acid and beta blockers.
The diabetic nephropathy in insulin-dependent diabetes mellitus
the Initial dose makes 6.25 mg/days (¼ tablets VIVA FARM Captopril). If necessary the dose is increased to 75-100 mg/days by 2-3 times a day. In insulin-dependent diabetes with a microalbuminuria (discharge of albumine of 30-300 mg a day) the dose of drug makes 50 mg twice a day. At the general clearance of protein more than 500 mg a day drug is effective in a dose of 25 mg three times a day.
In a renal failure
As elimination of Captopril is carried out through kidneys, the dosage at patients with a renal failure has to be reduced or intervals between administrations of drug are increased.
At the accompanying therapy, diuretics, at patients with a heavy renal failure, recommend loopback diuretics (for example, furosemide), but not diuretics of a thiazide row.
The daily dose of Captopril depends on indicators of clearance of creatinine (see the table).
Clearance of creatinine (ml/min. / 1.73 m ²)
the Initial dose
(mg/day)
the Maximum dose
(mg/day)
& gt,
40 21 – 40 10 – 20
& lt, 10
25 mg – 50
mg 25 mg 12.5 mg 6.25 mg 150 mg 100 mg 75 mg 37.5 mg
Elderly patients
At patients of advanced age the initial dose makes 6.25 mg (¼ tablets VIVA FARM Captopril) two times a day.
The elderly patient the dose of drug is selected individually, considering a possibility of a renal failure or other bodies.
Side effects
Often
– dizziness, sleep disorders
– dryness in a mouth, disturbance of flavoring feelings (the metal or salty smack independently disappearing in 2-3 months after an initiation of treatment), nausea, vomiting, an abdominal pain, diarrhea, a constipation
– a hyponatremia
– dry cough
– skin rash, an itching
Infrequently
– tachycardia, heartbeat, stethalgias
– arterial hypotension
– feeling of fatigue, an asthenia
Seldom
– anorexia
– increase in content in blood serum of creatinine, residual nitrogen of urea
– a renal failure, a polyuria, an oliguria, a proteinuria, the speeded-up urination
– a headache, drowsiness, paresthesias
– stomatitis, aphthous ulcers of a mucous membrane of a mouth
Very seldom
– a nephrotic syndrome
– a neutropenia, an agranulocytosis, a pancytopenia, anemia, thrombocytopenia, an eosinophilia, a positive caption of antinuclear antibodies, increase in SOE
– a hyperpotassemia, a hypoglycemia
– a bronchospasm, rhinitis, an allergic alveolitis / eosinophilic pneumonia
– a glossitis, stomach ulcer, pancreatitis
– an abnormal liver function, a cholestasia, jaundice, hepatitis, necrosis of hepatic cells, increase in level of hepatic transaminases, bilirubin
– a Quincke’s disease, Stephens-Johnson’s syndrome, a multiformny erythema, a photosensitization, exfoliative dermatitis
– myalgias, arthralgias
– impotence,
the Contraindication gynecomastia
– hypersensitivity to any component of drug and other inhibitors (AKF)
– a Quincke’s disease in the anamnesis or other vascular hypostases (for example, owing to the previous therapy by AKF inhibitors)
– the profound renal failures (clearance of creatinine less than 30 ml/min.) or a liver
– a hyperpotassemia
– a leukopenia, thrombocytopenia
– the desensibilizing therapy against poisons of insects (life-threatening anaphylactoid reactions – falling of arterial blood pressure, suffocation, vomiting, allergic skin reactions)
– dialysis with use polyacrylonitrile-methallyl-sulfonatnykh of membranes with high-permeability (for example, “AN 69″ can develop) because of danger of development of anaphylactoid reactions (reaction of hypersensitivity), up to shock
– a bilateral stenosis of renal arteries or a stenosis of an artery of the only kidney with the progressing azotemia
– a state after transplantation of a kidney
– the stenosis of the mouth of an aorta and similar changes complicating blood outflow
– primary hyper aldosteronism
– pregnancy and the period of a lactation
– children’s and teenage age up to 18 years
Medicinal interactions
Diuretics, vazodilatator, ganglioblokator, adrenoblockers strengthen hypotensive effect of captopril.
Indometacin and other non-steroidal anti-inflammatory drugs and also a clonidine can reduce antihypertensive effect of captopril.
Simultaneous use with kaliysberegayushchy diuretics (for example, Triamterenum, Spironolactonum, amiloride), potassium drugs, cyclosporine, milk with low the content of salts (may contain K+ to 60 mmol/l), potassium additives, salt substitutes (contain significant amounts of K+) increases risk of development of a hyperpotassemia.
At the patients accepting Allopyrinolum and prokanainamid or immunodepressants (for example, Azathioprinum and cyclophosphamide) in a combination with captopril the risk of development of hematologic disturbances, neutropenias and/or a syndrome Stephens-Johnson increases.
Removal of captopril through kidneys decreases at Probenetsid’s appointment.
Simultaneous use of salts of lithium and inhibitors can lead the angiotensin-the converting enzyme (ACE) to increase in concentration of lithium in blood serum. At the same time the risk of emergence of side and toxic effects of drugs of lithium increases.
Captopril reduces hypoglycemic effect of antidiabetic drugs (insulin, sulphonylurea derivatives), causes a hyperglycemia.
The special
instructions Development of Symptomatic Arterial Hypotension perhaps at patients with a hyponatremia and/or with a reduced volume of the circulating blood in the result of treatment with diuretics, uses of special diets or dehydration of an organism for other reasons (profuse sweating, repeated vomiting, a diarrhea, dialysis) and in heart failure. Treatment of hypotension consists of providing a bed rest and, in case of need, infusional therapy. The passing lowering of arterial pressure is not a contraindication for treatment by captopril, however, temporary drug withdrawal or a dose decline can be required. Captopril normalization of a water and electrolytic imbalance and elimination of deficit of volume of the circulating blood by all means has to precede treatment, besides, observation of change of arterial blood pressure after reception of an initial dose is necessary.
In cerebrovascular diseases and coronary heart disease it must be kept in mind that the considerable lowering of arterial pressure can lead to development of a stroke or myocardial infarction.
Renovascular hypertensia, a stenosis of renal arteries (bilateral or unilateral with one kidney)
At some patients with renovascular hypertensia, with a bilateral stenosis of renal arteries or a stenosis of an artery of the only kidney is present when prescribing captopril the increased risk of developing heavy arterial hypotension and renal failure (concentration of urea and creatinine in blood serum increases). At these patients the treatment by captopril should be begun under stringent medical control with small doses with the subsequent titration, regular monitoring of function of kidneys is necessary.
A stenosis of aortal, mitral valves, a hypertrophic cardiomyopathy
AKF Inhibitors should be applied with care at patients with a stenosis of aortal, mitral valves or a hypertrophic cardiomyopathy.
Cough
Cough when prescribing AKF inhibitors is unproductive, resistant and passes after the therapy termination.
The Quincke’s disease
the Quincke’s disease of the face, extremities, lips, language, a glottis and/or throat is observed seldom at the patients receiving AKF inhibitors. This complication can occur at any time during therapy by captopril. In such cases administration of drug it is necessary to stop and appoint immediately the corresponding treatment before full elimination of clinical symptoms of hypostasis.
The neutropenia, an agranulocytosis
the Neutropenia, an agranulocytosis, thrombocytopenia and anemia are observed seldom or never at the patients receiving AKF inhibitors. These phenomena are reversible after the termination of intake of captopril. Drug should be used with extra care at patients with the autoimmune diseases receiving immunodepressants, Allopyrinolum or procaineamide. At some of these patients development of serious infections which in certain cases do not respond to intensive antibacterial care is possible. When using captopril at such patients the control of level of leukocytes in blood prior to treatment, then each 2 weeks within the first 3 months of administration of drug and periodically further is recommended.
A liver failure
Very seldom AKF inhibitors cause cholestatic jaundice or hepatitis, up to hepatocellular necrosis. The mechanism of these complications is not known. Patients at whom jaundice or increase in level of liver enzymes develops should stop intake of captopril and to receive the corresponding therapy.
Hemodialysis / LPNP-lipidny-aferez / Therapy for desensitization
At co-administration of captopril and dialysis about polyacrylic – a nitrile membrane or LDL (lipoprotein of low density) – an afereza by means of sulfate of a dextran or desensitization against poisons of insects (bees, wasps) the development of an acute anaphylaxis is possible. It is recommended to use other membrane for dialysis or it is necessary to replace temporarily captopril with other antihypertensive drugs (not AKF inhibitors). Before performing desensitization it is necessary to cancel captopril.
Surgical intervention / anesthesia
Can be observed arterial hypotension at extensive surgeries or in case of use of drugs with hypotensive effect. Hypotonia can be eliminated by completion of volume of the circulating blood.
Patients with diabetes
It is necessary to control carefully glycemia level at the patients with diabetes who were earlier receiving oral anti-diabetic drugs or insulin namely within the first month of treatment by captopril.
Hereditary intolerance of a galactose, deficit of Lapp of lactase, a syndrome of the broken glucose absorption – a galactose
patients should not appoint Captopril with seldom observed hereditary intolerance of a galactose, deficit of Lapp of lactase or a syndrome of the broken glucose absorption.
The care at control of vehicles or performance of other work requiring special attention as dizziness, especially after an initial dose of captopril is possible Is necessary for feature of influence of medicine on ability to run the vehicle or potentially dangerous mechanisms.
Overdose
Symptoms: the profound hypotension, with possible development of a myocardial infarction, acute disorder of cerebral circulation and tromboembolic episodes against the background of a sharp lowering of arterial pressure.
Treatment: the patient should accept horizontal position (legs have to be raised) and to enter to it intravenously isotonic solution of sodium of chloride or other plasma substituting liquids for correction of the volume of the circulating blood (VCB). Captopril is removed from an organism by means of a hemodialysis.
The form of release and packing
On 15 tablets place in blister strip packaging from a film of polyvinylchloride and printing aluminum foil.
On the 2nd blister strip packagings together with the instruction for medical use in the state and Russian languages put in a box of cardboard.
To Store storage conditions at a temperature not over 25 °.
To store out of children’s reach!
Period of storage
3 years.
Not to use drug after the expiry date specified on packing
Prescription status
According to the prescription
VIVA FARM LLP Producer,
Republic of Kazakhstan 2nd Ostroumova St., 33, Almaty, 050030
ph.: +7 (727) 383 74 63, fax: +7 (727) 383 74 56
The name and the country of the owner of the registration certificate
of VIVA FARM LLP, the Republic of Kazakhstan
the Address of the organization accepting in the territory of the Republic of Kazakhstan claims from consumers on quality of products
TOO « VIVA PHARM” 2nd Ostroumova St., 33, Almaty, 050030, Republic of Kazakhstan
ph.: +7 (727) 383 74 63, fax: +7 (727) 383 74 56,
e-mail:
To Develop pv@vivapharm.kz
Additional information
| Ingredient |
|---|















Reviews
There are no reviews yet.